
Report of an Ad Hoc Panel of the Advisory Committee on Technology Innovation Board on Science and Technology for International Development Office of International Affairs National Research Council
NATIONAL ACADEMY PRESS
Washington, D.C. 1983
NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the Councils of the National Academy of Sciences, the National Academy of Engineering, and the Institute of Medicine. The members of the Committee responsible for the report were chosen for their special competences and with regard for appropiate balance.
This report has been reviewed by a group other than the authors according to procedures approved by a Report Review Committee consisting of members of the National Academy of Sciences, the National Academy of Engineering, and the Institute of Medicine.
The National Research Council was established by the National Academy of Sciences in 1916 to associate the broad community of science and technology with the Academy's purposes of furthering knowledge and of advising the federal government. The Council operates in accordance with general policies determined by the Academy under the authority of its congressional charter of 1863, which establishes the Academy as a private, nonprofit, self-governing membership corporation. The Council has become the principal operating agency of both the National Academy of Sciences and the National Academy of Engineering in the conduct of their services to the government, the public, and the scientific and engineering communities. It is administered jointly by both Academies and the Institute of Medicine. The National Academy of Engineering and the Institute of Medicine were established in 1964 and 1970, respectively, under the charter of the National Academy of Sciences.
The Board on Science and Technology for International Development (BOSTID) of the Office of International Affairs addresses a range of issues arising from the ways in which science and technology in developing countries can stimulate and complement the complex processes of social and economic development. It oversees a broad program of bilateral workshops with scientific organizations in developing countries and conducts special studies. BOSTID's Advisory Committee on Technology Innovation publishes topical reviews of technical processes and biological resources of potential importance to developing countries.
This report has been prepared by an ad hoc advisory panel of the Advisory Committee on Technology Innovation, Board on Science and Technology for International Development, Office of International Affairs, National Research Council. Program costs for the study were provided by the Office of Technical Resources, Bureau for Asia, Agency for International Development, under Grant No. ASB-0249-SS-00-1026-00 and the Office of the Science Advisor, Agency for International Development, under Grant No. DAN/ 5538-G-SS-1023-00.
Funding for this printing was provided by the Office of Agriculture, Bureau for Science and Technology, Agency for International Development, Washington, D.C., under Grant No. DAN 1406-G-SS-4001-00.
First Printing, June 1983
Second Printing, July 1984
Library of Congress Catalog Card Number 83-061909
Participants in the Study
HUGH POPENOE, Director, International Programs in Agriculture, University of Florida, Gainesville, Florida, USA, Chairman
Contributors
AMBAR ROESYAT, Research Institute for Animal Husbandry, Bogor, Indonesia
1. B. ARKA, Faculty of Veterinary Science and Animal Husbandry, Udayana University, Bali, Indonesia
J. S. F. BARKER, Professor of Animal Sciences and Head of Department, The University of New England, Armidale, New South Wales, Australia
KURT BENIRSCHKE, Research Department, San Diego Zoological Garden, San Diego, California, USA
BEN BERESKIN, Research Geneticist, Nonruminant Animal Nutrition Laboratory, Animal Science Institute, United States Department of Agriculture, Beltsville, Maryland, USA
RALEIGH A. BLOUCH, World Wildlife Fund, Bogor, Indonesia
W. BONGERS, Nature Conservation Department, Wageningen Agricultural University, Wageningen, The Netherlands
I. BONNEMAIRE, Ecole Nationale Superieure des Sciences Agronomiques Appliquees, Dijon, France
A. A. BOSMA, Vakgroep Funktionele Morfologie, Rijksuniversiteit, Utrecht, The Netherlands
D. BUTCHER, Assistant Director, Western Plains Zoo, Dubbo, New South Wales, Australia
G. S. CHILD, Wildlife and Parks Management Officer, Food and Agriculture Organization, Rome, Italy
W. ROSS COCKRILL, International Animal Consultant, Almansil, Algarve, Portugal
PAUL CONRY, Division of Aquatic and Wildlife Resources, Department of Agriculture, Guam
HAROLD J. COOLIDGE, Former President, International Union for the Conservation of Nature, Beverly, Massachusetts, USA
W. P. CROWCROFT, General Director, Metropolitan Zoo, Toronto, Canada
TONY J. CUNHA, Dean Emeritus, California State Polytechnic University, Pomona, California, USA
D. DEPPNER, Tropical Livestock Consultant, Washington, D.C., USA
C. DEVENDRA, Head, Feed Resources and Animal Nutrition Branch, Malaysian Agricultural Research and Development Institute, Selangor, Malaysia
J. M. DOLAN, San Diego Zoological Garden, San Diego, California, USA DUKUT SULARSASA, Department of Tropical Veterinary Science, James Cook University, Townsville, Queensland, Australia
H. EPSTEIN, Faculty of Agriculture, The Hebrew University of Jerusalem, Tel Aviv, Israel (retired)
T. FINNIE, Taronga Zoo, Mosman, New South Wales, Australia
H. FISCHER, Director, Institute of Tropical Veterinary Medicine, JustusLiebig-Universitat, Giessen, Federal Republic of Germany
IAN FLETCHER, Project for Animal Research and Development, Bogor, Indonesia
ANNIE P. GRAY, Commonwealth Bureau of Animal Breeding and Genetics, Edinburgh, Scotland (retired)
R. B. GRIFFITHS, Director, Animal Production and Health Division, Food and Agriculture Organization, Rome, Italy
1. R. GRiMWOOD, Nairobi, Kenya
COLIN P. GROVES, Australian National University, Canberra, A.C.T., Australia
ULRICH HALDER, Swiss League for Nature Conservation, Basel, Switzerland
CHARLES G. HICKMAN, Livestock Consultant, A. Mithat Efendi Sokak No. 36/11, Cankaya, Ankara, Turkey
D. HOFFMAN, Veterinarian, Project for Animal Research and Development, Bogor, Indonesia
J. H. G. HOLMES, Department of Agriculture and Forestry, University of Melbourne, Victoria, Australia
H. HUITEMA, Veterinarian, Osterbeek, The Netherlands
J. H. HUTASOIT, Director-General, Livestock Services, Jakarta, Indonesia
BENT JORGENSEN, Director, Copenhagen Zoo, Copenhagen, Denmark
NAT KlEFFER, Department of Animal Science, Texas A&M University, College Station, Texas, USA
F. WAYNE KING, Director and Professor, Florida State Museum, University of Florida, Gainesville, Florida, USA
GRAHAM W. M. KIRBY, Principal Animal Production Officer, Department of Primary Production, Darwin, Northern Territory, Australia
HEINZ-GEORG KLOS, Director, Zoologischer Garten, Berlin, West Germany
KUSMAT TANUDIMADJA, Professor of Veterinary Anatomy, Faculty of Veterinary Medicine, Institut Pertanian, Bogor, Indonesia
JOHN K. LOOSLI, Department of Animal Science, University of Florida, Gainesville, Florida, USA
JOHN A. LUKAS, White Oak Plantation, Yulee, Florida, USA
A. A. MACDONALD, Vakgroep Funktionele Morfologie, Rijksuniversiteit, Utrecht, The Netherlands
JOHN MACKINNON, Representative, World Wildlife Fund, Bogor, Indonesia
ROBERT E. McDOWELL, Professor, Department of Animal Science, Cornell University, Ithaca, New York, USA
JEFFREY A. McNEELY, Executive Officer, Commission on National Parks and Protected Areas, International Union for Conservation of Nature and Natural Resources, Gland, Switzerland
ADRIAN G. MARSHALL, Honorary Secretary, Institute of South-east Asian Biology, Department of Zoology, University of Aberdeen, Scotland
[AN L. MASON, Animal Breeding Consultant, Edinburgh, Scotland
GEERT MONTSMA, Vakgroepen Veefokkerij, Veehouderij, Tropische Veehouderij, Wageningen, The Netherlands
J. B. MORAN, Senior Research Officer, Animal and Irrigated Pastures Research Institute, Kyabram, Victoria, Australia
ROBERT H. MILLER, Chief, Milk Secretion and Mastitis Laboratory, Beltsville, Maryland, USA
JAN NARI, Director, Central Research Institute for Animal Science, Bogor, Indonesia
HARVEY NEESE, President, Agri-Food Systems International, Inc., Troy, Idaho, USA
I. M. NITIS, Department of Animal Nutrition and Tropical Pasture Production, Udayana University, Denpasar, Bali, Indonesia
WILLIAM L. R. OLIVER, Chairman, lUCN/SSC Pigs and Peccaries Specialist Group, Jersey, Channel Islands, United Kingdom
JOHN PAYNE, Wildlife Section, Forest Department, Sandakan, Sabah, Malaysia
W.J.A. PAYNE, Tropical Animal Breeding Consultant, London, England
RALPH W. PHILLIPS, Arlington, Virginia, USA
DONALD L. PLUCKNETT, Scientific Advisor, Consultative Group on International Agricultural Research, Washington, D.C., USA
DAVID W. ROBINSON, Associate Dean, International Programs, University of California, Davis, California, USA
PATRICK J. ROBINSON, Department of Agricultural and Forest Sciences, University of Oxford, Oxford, England
D. H. L. ROLLINSON, Animal Production Officer, Animal Production and Health Division, Food and Agriculture Organization of the United Nations, Rome, Italy
CLIVE ROOTS, Director, Assiniboine Park Zoo, Winnipeg, Manitoba, Canada
J. A. SAYER, Nature Conservation and National Parks Project, FAO, Rangoon, Burma
R. E. SATTER, Nature Conservation and National Parks Project, FAO, Rangoon, Burma
GEORCE B. SCHALLER, New York Zoological Society, New York, New York, USA
JOHN SCHOTTLER, Principal Animal Production Officer, Department of Primary Industry, Lae, Papua New Guinea
GEORGE SEIFERT, CSIRO, Rockhampton, Queensland, Australia
ROGER V. SHORT, Professor of Reproductive Biology, Monash University, Melbourne, Australia
C. C. SINHA, Outdoor Recreation and Wildlife Research Division, Forest Research Institute, College, Laguna, Philippines
FREDERICK J. SIMOONS, Professor, Department of Geography, University of California, Davis, USA
C. D. SIMPSON, Senior Extension Specialist, Department of Agricultural, Technical and Extension Services, Bulawayo, Zimbabwe
P. SITORUS, Research Institute for Animal Production, Bogor, Indonesia
A. J. SMITH, Royal School of Veterinary Studies, University of Edinburgh, Edinburgh, Scotland
D. J. STOLP-DIEPEVEEN, Department of Tropical Animal Production, Agricultural University, Wageningen, The Netherlands
SUBANDRlYO, Research Institute for Animal Production, Bogor, Indonesia
W. SUMADI, Faculty of Animal Husbandry, Gadjah Mada University, Yogyakarta, Indonesia
L. M. TALBOT, World Wildlife Fund International, Gland, Switzerland
WARREN D. THOMAS, Director, Los Angeles Zoo, Los Angeles, California, USA
J. L. THROP, Director, Taronga Zoo, Mosman, New South Wales, Australia
ALLEN D. TILLMAN, Private Consultant in Animal Production, Stillwater, Oklahoma, USA
DONALD G. TULLOCH, Division of Wildlife Research, CSIRO, Winnellie, Northern Territory, Australia
HELEN NEWTON TURNER, Genetics Research Laboratories, CSIRO, North Ryde, New South Wales, Australia
B. VAN PUIJENBROECK, Curator of Mammals, Royal Zoological Society of Antwerp, Antwerp, Belgium
E. J. WARWICK, Visiting Professor, Faculty of Animal Husbandry, Gadjah Mada University, Yogyakarta, Indonesia
WARTOMO HARDJOSUBROTO, Lecturer in Animal Husbandry, Faculty of Animal Husbandry, Gadjah Mada University, Yogyakarta, Indonesia
R. H. WHARTON, Chief Research Scientist, CSIRO, Long Pocket Laboratories, Brisbane, Australia
J. L. WHEELER, Project Manager, Project for Animal Research and Development, Bogor, Indonesia
M.H. WOODFORD, Wildlife Veterinarian, Food and Agriculture Organization, Rome, Italy
B.A.YOUNG, Department of Animal Science, the University of Alberta, Edmonton, Canada
M.ZULBARDI, Research Institute for Animal Production, Bogor, Indonesia
NOEL D. VIETMEYER, Professional Associate, Board on Science and Technology for International Development, Asian Animals Study Director
National Research Council Staff
F. R. RUSKIN, BOSTID Editor
MARY JANE ENGQUIST, Staff Associate
CONSTANCE RECKS, Administrative Secretary
It is high time that the world was made aware of the valuable, large ruminant genetic resources of Southeast Asia.
J. B. MORAN Livestock Specialist Animal and Irrigated Pastures Research Institute Kyabram, Victoria, Australia
Without utilization preservation is doomed to failure. Local breeds able to perform well in difficult habitats, such as in sparse vegetation, mountainous terrain, or the tropics, should be used for their agricultural potential. Breeds that demonstrate hybrid vigor on crossing with improved or exotic breeds also merit utilization. Genetically unique breeds should be retained for scientific studies of genetics, evolution, and biochemistry. And aesthetically attractive and historically important breeds should be maintained in parks and preserves for their educational and cultural values.
I. L. MASON Animal Breeding Consultant Edinburgh. Scotland

This report began as a brief examination of the domesticated banteng, a little studied bovine that is an important livestock resource in eastern Indonesia. But the study expanded to include other possibly useful but obscure bovines of Asia: the madura (a hybrid between banteng and cattle), gaur, mithan, kouprey, anoa, tamaraw, yak, and yakows—hybrids formed by crossing yaks with cattle. Four pig species were also included (the bearded pig, the Sulawesi warty pig, the Javan warty pig, and the pygmy hog) that are important traditional resources in parts of Asia, as well as the babirusa—a piglike, wild animal of Sulawesi that may have a rudimentary rumen. (Asia also has some interesting, little-known breeds of cattle, water buffalo, sheep, goats, and the common pig, but in this report we have chosen to highlight unconventional species only.)
These animals are unfamiliar to international animal science. This report aims to kindle awareness of their possible promise and to stimulate their introduction into projects in the world's animal research facilities. Only through active investigation will the potential of these species - most of which are now familiar only to a small number of wildlife specialists - become clear. Many are threatened with extinction; their genetic merits should be assessed before it is too late.
Development agencies and governments in the tropics should regard local species, such as those described in this report, as important genetic material for bolstering the long-term success of domestic livestock breeding programs. These indigenous resources can probably be improved genetically to compare favorably with imported livestock, particularly in difficult tropical environments.
This report was prepared after Hugh Popenoe, Chairman of the Advisory Committee on Technology Innovation (ACTI), and Noel Vietmeyer of the ACT! staff visited banteng researchers in Indonesia, northern Australia, and Papua New Guinea in May 1981. More than 90 researchers (see contributor's list) provided information to the report through correspondence.
ACTI is a committee of the Board on Science and Technology for International Development, National Research Council. It assesses unconventional resources and technologies that might prove especially applicable to problems of developing countries. Current titles in the ACTI series Managing Tropical Animal Resources are:
· Water Buffalo: New Prospects for an Underutilized Animal (1981)
· Little-Known Asian Animals with a Promising Economic Future (1983)
· Crocodiles as a Resource for the Tropics (1983)
· Butterfly Farming in Papua New Guinea (1983).
The production of these books has been supported largely by the U.S. Agency for International Development (AID). Program costs for this study were sponsored by AID'S Bureau for Asia, and staff costs by AID'S Office of the Science Advisor, which also made possible the free distribution of this report.
This is an exploratory volume. It will, perhaps, lead in a few years' time to a more complete book on indigenous Asian animals - and perhaps also to similar books on little-known animals of Africa and Latin America. If you have material to contribute to the future edition, please send it to Noel Vietmeyer, JH213, National Academy of Sciences, 2101 Constitution Avenue, Washington, D.C. 20418, USA, who will contact you when any new publication on this subject is planned.
Asia has several domesticated animals about which little is known. Among them are the banteng ("Bali cattle") of Indonesia, the yak of Central Asia's high country, and the mithan of the border region of India, Burma, and Bangladesh.( *The promise of another Asian animal, the water buffalo, is described in companion report no 32. ) Some Asian farmers use domesticated bovine hybrids: the madura (banteng-cattle hybrid) in Indonesia and the yakow (yak-cattle hybrid) in Central Asia. In addition, domesticated forms of at least two Asian pig species (the Indonesian wild boar and the Sulawesi warty pig) are important husbandry animals in parts of Indonesia.
Among Asia's undomesticated animals are five interesting and potentially valuable species of wild bovines: the kouprey in Thailand, Laos, and Kampuchea; the gaur in India and much of Southeast Asia; two species of anoas in Indonesia; and the tamaraw in the Philippines. There are also three species of undomesticated Asian pigs: the bearded pig, the Javan warty pig and pigmy hog. Perhaps the strangest Asian animal of all is the babirusa, a piglike species of eastern Indonesia that may be a rudimentary ruminant.
Many of these animals are either threatened or endangered species, and some will soon be extinct unless scientists, governments, and resource managers take forceful action to preserve them. Scientific management and a better understanding of the animals themselves is required.
An important need is to investigate their potential as livestock resources. All seem to be disease resistant and well adapted to difficult natural conditions. Some will interbreed with conventional livestock and might thereby pass on important characters to hybrid progeny. A few of the wild species are ancestors of domestic livestock and could be important genetic reservoirs for maintaining or improving the quality of their domestic descendants. Others may make useful new domesticates.
Appreciation of their potential in the long-term for the world's agricultural development could create a momentum for the protection, preservation, and greater use of these little-known animals.
Imported Livestock
In the past, many thought that the best way to raise animal productivity in tropical developing countries was to introduce high-performing breeds from temperate industrialized areas. The fact that the exotic animals were much more productive than local stock made this seem very appealing. But many such importations ended in failure when the animals either quickly died or their growth or reproduction rates declined so drastically that the herds dwindled away or became uneconomic. These introductions had been made without adequate consideration of the local environment that was expected to support the imported animal.
More recently, some animal scientists have decried the idea of wholesale importing of temperate-zone livestock into tropical countries without also evaluating the indigenous livestock. In many cases the local animals' apparent poor performance results not from their lack of genetic potential, but from inadequate feeding, breeding, selection, management, and health care.
Indigenous Animals
Until an objective evaluation of particular indigenous breeds is undertaken, governments should regard local animals as vitally important for the long-term development of their domestic livestock industries.
To achieve an agriculture that is compatible with nature, we should try to raise an animal as much as possible within its own natural environment. Indigenous species are necessarily well adapted to their surroundings and have survival qualities that imported livestock often lack. They generally select food, either graze or browse, better than foreign species and can therefore exploit the habitat more efficiently and live within it more harmoniously.
But most of the livestock species the world depends on today are best adapted to temperate conditions. This is because Stone Age peoples in what today are Europe, the Middle East, and eastern China domesticated the prototypes of agricultural animals that were available in those places. The world's best-known breeds of cattle, wool sheep, and horses, for example, usually perform well in temperate regions and poorly in the tropics.
The species described in this report offer promise as new and important alternative livestock resources for tropical regions.
Embryo Transplants
It seems likely that modern technology will substantially improve our ability to use new species and breeds of livestock. One of the most encouraging of the new biotechnology techniques is embryo transplantation. In this process multiple eggs (produced by hormonally stimulating female animals) are fertilized and transplanted to other female animals. The hormonal cycles of the donor and receiving animals are synchronized so that a pregnancy results.
This technique is widely used to transfer embryos between cattle, but researchers are now exploring transfers between different species and even between different genera. In 1981, for example, animal scientists at the New York Zoological Garden transplanted a fertilized gaur ovum into a Holstein cow, which carried the gaur calf to term. Now veterinarians at the University of Florida are attempting to transplant water buffalo embryos into both zebu and Holstein-Friesian cattle, and vice versa. If such pioneering work can become everyday practice, it will open possibilities of using common livestock to raise rare animals.
Moreover, because embryos are thought to be free of many diseases, their shipment between nations may soon be permitted without elaborate quarantine precautions. Methods for freezing embryos have been worked out so that the tiny bundles of cells can be air freighted inexpensively in small, insulated containers. This may make animals such as those discussed in this report available to Africans, Latin Americans, North Americans, and Europeans. It may vastly simplify the worldwide exchange of animal genetic material and become the animal counterpart of exchanging seeds.
An additional benefit of embryo transplants is that the surrogate mother's placental blood supply provides the fetus with natural immunity to some local diseases, thus perhaps reducing one of the most serious causes of failure when exotic animals are introduced to new environments. And at Utah State University it has been noted that raising wildsheep embryos in domestic ewes produces lambs that are more docile than if they were raised by their biological mothers. Embryo transplants may be a small step toward domesticating new animals.
With these possibilities on the horizon, it becomes even more important that all countries preserve their indigenous animals. The rest of this report suggests and discusses Asian species for protection and study.
It seems probable that two of our widely used livestock species were domesticated in the Asian tropics: the zebu or humped cattle in India and the water buffalo in the humid marshlands of northeastern India or Southeast Asia. (Both the chicken and some races of pig may also have a tropical Asian origin, but the exact sites of their earliest domestication is unclear.) There are, however, other, much more localized, domesticated bovines in Asia. These are not well studied and deserve greatly increased recognition.
This section highlights:
· Domesticated banteng
· Banteng-cattle hybrids
· Mithan
· Yak
· Yakows.
It is time for the world's scientific community to study the genetics, evolution, and biochemical parameters of the unique animal resources, that are found in Southeast Asia. ALLEN D. TILLMAN Animal Consultant Stillwater, Oklahoma, USA
Though well adapted for survival in their own harsh tropical environments, many domestic livestock breeds are being neglected in the race to achieve temperate zone levels of productivity. CHARLES HICKMAN Department of Dairy Cattle Breeding Ottawa, Canada
Studies should be made of the present and potential role, productivity and efficiency of all domestic animals and birds, large and small, before they are replaced by imported types. Many wild species also could make important contributions to human welfare with proper management. J. K. LOOSLI Department of Animal Science University of Florida .
The conservation of endangered genetic resources represents a genuine and welcome synthesis of the concerns of the agriculturalist and the wildlife enthusiast.
JOHN TINKER British Journalist

The banteng (*Bos javanicus is now the accepted name, but Bos sondaicus, Bibos banteng, and other synonyms have been used in the past. (See Hooijer, 1956.) The name "banteng" has traditionally referred to the wild form of Bos javanicus; the name "Bali cattle" to the domesticated form. This chapter describes the domesticated form, but we retain the name banteng to reinforce the fact that the animal is not a breed of cattle, but a distinct species. Despite a cattle-like appearance, the animals are at least as genetically remote from cattle as is the bison. Both produce sterile males when hybridized with European cattle. Australia. (N.D. Vietmeyer)) is a bovine that resembles a small cow. It is, however, an entirely different species from either European cattle (Bos taurus) or zebus (Bos indicus).
These docile animals thrive under hot, humid conditions and, like the water buffalo, have high resistance to ticks and tick-borne diseases. In parts of Southeast Asia they have proved acceptable for draft power and meat production, and they may have potential for many other regions of the world.
Appearance and Size
Banteng are remarkably uniform in type and have changed little from their wild ancestors (see chapter 6). Bulls stand from 1.3 to 1.5 m high at the shoulders, while cows are about 1.2 m high. They have the general conformation of beef cattle. The skin is tight, the neck short, and the dewlap inconspicuous. The face is narrow and is carried horizontally with the large ears pointing forward. There is no hump, but the male possesses a distinctive crest over the thorax because the spines of the thoracic vertebrae are unusually elongated.
On both sexes a striking white oval patch covers the rump and all four legs have white stockings. Calves and females are usually light brown with a thin black line along the middle of the back. Bulls are brown when young but usually turn near-black at maturity, unless castrated. Thus, in a herd the sexes - black males and brown females - are strikingly apparent.
Male horns grow sideways, upward, and outward from a horny mass on the forehead. Females lack this horny shield and their horns usually grow upward and back, eventually curving down again toward the head. Polled animals have not been reported.
Mature banteng bulls can weigh from 450 to 500 kg. Under exceptional conditions they may reach 550 kg.
Distribution
Nearly all the world's domesticated banteng are found in Indonesia. They are particularly important on the islands of Bali, Kalimantan, Lombok, Sulawesi, Sumbawa, and Timor. On Bali and Sumbawa, they are virtually uncontaminated by crossbreeding with other cattle and are thought to have been domesticated there in prehistoric times. Since 1913 government officials have enforced a law that prohibits crossbreeding so as to maintain the purity of the breed.
Small numbers of banteng have been introduced to Sumatra, Malaysia, and northern Australia, and there are experimental herds in Texas, USA, and New South Wales, Australia.
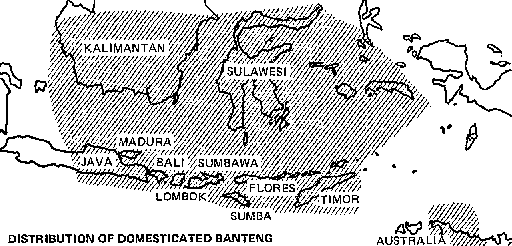
Status
Domesticated banteng account for about 20 percent of Indonesia's total population of "cattle." The banteng population increased from 1.1 million in 1967 to 1.4 million in 1975 and is now estimated to be more than 1.5 million.
Habitat and Environment
Banteng are found mainly in lowland areas on Indonesian islands straddling the equator. The environment is monsoonal with mean annual temperatures ranging from 23° to 31°C. Most of the region receives heavy rainfall throughout the year, but dry spells as long as five months occur at some locations.
Biology
Banteng normally have high conception rates. In northern Australia they regularly achieve 80-90 percent conception, as compared with the 50-60 percent of Brahman-Shorthorn crossbred cattle. Similar conception rates have been recorded in Sulawesi.
Little basic information on the banteng's reproductive physiology has been reported. However, it appears that this differs little from that of cattle. Sexual maturity has been observed as early as 13 months and mating at 16 months.* The gestation period is about one week longer than that of cattle and twinning is not common. At birth, males weigh about 16-17 kg and females about 14-15 kg (about half the size of Brahman-Shorthorn calves). Calves are not weaned at any specific time, and dams may continue suckling a calf until the next birth.
Banteng will crossbreed with domestic cattle, producing hybrids with notable vigor and heat tolerance (see chapter 2).
It has been reported that banteng have the ability to drink water with high salinity," and in northern Australia they have even been seen grazing seaweed on coral reefs at low tide.
On high-quality diets, feed-conversion ratios approach those of other bovine species. For example, banteng bulls on high-concentrate diets have shown daily growth rates of 0.7 kg, compared with 0.7 kg in water buffalo, 0.8 kg in zebu cattle, and 0.9 kg in British cattle bulls.:"
As the banteng matures, its weight remains lower than that of cattle of similar age. However, relative to mature liveweight, this disadvantage is only about 8 percent.§
Behaviour
Banteng cannot be handled as roughly as domestic cattle. Cows and calves are timid and easily upset. When stressed they may run into fences and walls, incurring head and spinal injuries. They also easily get into a state of shock. Special squeeze chutes and other special facilities, along with much care in handling the animals, are essential. However, despite their lively temperament banteng are docile if reared, as in Indonesia, with frequent human contact.
Uses
These animals are promising beef producers. Gourmets consider banteng cuts among the finest of meats, and Indonesia cannot export enough to satisfy the demand in Hong Kong and Japan alone. The meat's outstanding characteristics are its tenderness and leanness. When the animals are maintained and finished under traditional village management, total fat content of the meat (both on a liveweight and carcass basis) is usually less than 4 percent. Little of the fat is deposited among the meat fibers (marbling); about two-thirds of it is mesenteric and the remainder is subcutaneous, laid down in small globules. The carcasses have a high dressing-out percentage.
Banteng have proved to be useful as draft animals for the farm but are reportedly less suitable than zebus for hauling carts on roads. Traditionally, both male and female animals are worked, but Balinese farmers normally use females for cultivating light soils only. Banteng are trained when they are about 2 years old and are reportedly easier to train than zebus.
Potential Advantages
Like water buffaloes banteng can utilize low-quality feeds; they are similar in that they can live off forage unpalatable to cattle. It is rare to see banteng in poor condition. Animals of all ages appear to have an ability to maintain weight and body condition even when pasture quality is poor. In this respect, they have been noted to outperform cattle in Australia's Northern Territory. Moreover, due to their lower milk production banteng cows lose less weight and condition during lactation than, for example, Brahman-Shorthorn cows.
In comparison with cattle (European breeds) kept under similar circumstances in Australia, banteng are less infested with external parasites. Their hair is short and their hide tough, which helps them resist ticks. Under field conditions few adult cattle ticks (Boophilus microplus) are observed on the animals, except on malnourished individuals, and the incidence of tick-borne disease is said to be very low. Sarcoptic mange is known to affect the animals, but it has a low incidence as well.
Banteng also appear to tolerate several internal parasites. Liver fluke is probably the most prevalent. Although the animals themselves appear healthy at slaughter, some 80 percent or more of carcasses are found to have partially infected livers. In addition, intestinal worms are often present.
The animals are only slightly affected by Asian trypanosomiasis (Trypanosoma evansi).
Limitations
The animals are poor milkers; their udders are almost invisible. The lactation varies from 6 to 10 months and milk production is only 0.9-2.8 kg per day.
To keep from reverting to the wild state, banteng may need close contact with humans or regular handling.
An unidentified banteng disease first appeared on Bali in 1964. Known locally as "jembrana," it spread rapidly, causing mortalities from 10 to 60 percent in certain localities. It was originally thought to be rinderpest; it is now believed to be caused by a rickettsia transmitted by cattle ticks. Outbreaks have occurred with decreasing intensity and virulence since the original outbreak. The disease is under intensive investigation.
"Bali ziekte," a disease affecting only banteng, has also been noted. It is characterized by dry eczema, followed by severe necrosis of the skin and exposed mucous membrances.
Because of their high susceptibility to bovine malignant catarrhal fever, banteng must not be brought in contact with sheep and some other animals. This virus is widely disseminated in sheep and several domestic and wild hoofed mammals.
Research and Conservation Needs
There have been few scientific studies of banteng and no coordinated attempt to improve their performance. They deserve more recognition and they merit testing in tropical locations outside their traditional home in Indonesia.
The herds in northern Australia (descendants of animals remaining from an abortive colonization attempt in the 1820s) are in a relatively disease-free zone, and although they are feral, they can potentially provide animals for breeding and use in other nations. Although these few hundred banteng are a valuable resource, they are vulnerable to destruction if adverse environmental conditions arise or exotic diseases break out in Australia. Representatives of the herd should therefore be distributed and viable breeding stock established at several new locations. (Their genes and gene combinations may even prove valuable for the indigenous herds in Indonesia, and banteng research and development would make a valuable topic for cooperative research between Australia and Indonesia.)
Research is particularly needed to identify the reasons for the banteng's apparent ability to maintain weight and body condition on poor-quality grazing.
The problems of bovine malignant catarrhal fever, Bali ziekte, and jembrana need increased attention, particularly directed toward prevention and control.
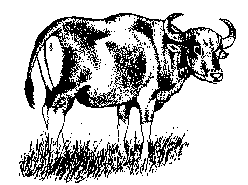
Banteng and cattle have the same number of chromosomes, and they will interbreed. Few scientific details on the hybrid progeny are available, but on the Indonesian island of Madura they are a "stabilized crossbreed" because they seem to be genetically uniform. This hybridization took place some 1,500 years ago, when Indian invaders brought zebus of the Sinhala, or Ceylonese, type to Madura and crossed them with the banteng.
These maduras† reportedly show better growth rate than the pure banteng species itself. They are thrifty, hardy, and able to perform well under extremes of heat and poor nutrition. Though a hybrid in origin, both sexes of the madura are fully fertile. ‡
For more than 15 centuries the winners of the bull races ("kerapan sap)") have been the herd sires of Madurese villages. This long breeding history has led to an animal with the following characteristics:
· Long legs and small feet
· Elongated muscles of the rear legs
· Heavy muscling over the back, loin, and shoulder
· Quick reactions and nervous temperament
· Great heat tolerance
· The ability to perform well as a work animal.
Appearance and Size
Banteng-cattle hybrids vary in appearance, depending on whether European or zebu cattle are used in the cross as well as on the amount of backcrossing.
Maduras (banteng x zebu) are graceful animals. Their bodies are neat, compact, and deep, with well developed forequarters. The cows attain an average weight of about 210 kg and bulls range from 350 to 375 kg at maturity.
Superficially, maduras are like Jersey cattle, except for having a much smaller udder. In most of them the banteng dominates the body structure and coat color. Bulls have a well-developed hump; females have almost none. There is no distinct dewlap. Horns are medium sized and curve upwards and slightly backwards. Ears are medium length and horizontal. Breeders on Madura accept only red-brown animals.
Distribution
Today, crossbreeds of banteng and zebu are distributed throughout Indonesia. On the other hand, hybrids between banteng and European cattle have been made only in small programs in the United States and Australia. For example, researchers in central Texas are producing a cross that is one-eighth banteng/seven-eighths Charolais. They believe this will result in a beef animal able to grow well in warm, humid conditions. In the Northern Territory of Australia, no problems have been encountered in mating banteng bulls to Brahman-Shorthorn cows. Calves that are one-fourth banteng/three-fourths Brahman-Shorthorn have since been produced.
Status
Virtually all of the 575,000 "cattle" on the Indonesian island of Madura are hybrid animals resulting from crossing indigenous domesticated banteng with zebus. More than 200 head of banteng/Charolais hybrids are found near Easterly, Texas, and a score or so banteng/ Brahman-Shorthorn hybrids are on a research station near Darwin, Australia.
Habitat and Environment
The environment in Madura, where most of these hybrids occur, is monsoonal, with mean average temperatures as high as 31°C in the hot season, and dry spells as long as 5 months. It seems likely that judicious selection of the cattle breed could result in hybrids suitable for many other environments.
Biology
As noted, the animals on Madura are bred to be raced. They are probably the fastest running bovines. From a standing start, a yoked pair pulling a sled can reach 50 kph by the end of the 130-meter course. An unencumbered individual can approach the speed of a horse (about 68 kph).
When compared with other bovine genotypes in Indonesia, the performance of these hybrids is more closely related to the banteng than the zebu ancestry."
In the cross between banteng and European cattle all F1 bulls are sterile; their sperm development ceases at the secondary spermatocyte stage.‡ Infertility also exists in most one-fourth banteng (although some sperm are present) and in all three-fourths banteng bulls. The fertility of the F1 cows, on the other hand, is high - 90 percent as compared with 70 percent for Brahman-Shorthorn cows.
Behavior
The hybrids have a lively temperament. However, when they are reared with other domestic cattle or handled on a regular basis, they are almost as docile as cattle. For instance, they remain calm even in Madura's extremely crowded towns. Nevertheless, personal contact probably must be maintained to keep them accustomed to human management.
The cows are very protective of their calves.
Uses
Several reports from the livestock service of the former Dutch colonial government, as well as several books on the East Indies, state that meat from the madura is the tenderest of any known breed. In addition, the hides are pliable and superior to those of cattle and are used in the highest quality leather goods. They command a price about 20 percent higher than zebu hides.
The breed is reportedly one of the best draft animals for its size in the world.
Potential Advantages
Maduras have several desirable traits, including those outlined below:
· Feed efficiency. With grain rations, yearling bulls can gain 1 kg on less than 7 kg of ration.
· Thriftiness. The breed can maintain its body condition on low quality forages.
· Heat tolerance. The madura has a high rate of cutaneous evaporation and is therefore well adapted to the tropical monsoonal climate. Teams of madura can plow or cultivate land for more than 6 hours, even at high temperatures.
· Carcass quality. The madura fattens readily on a high-quality diet and produces carcasses with high dressing percentages, a large rib-eye area, and high yields of lean meat.
· External fat thickness. A well-finished madura normally has only a slight covering of fat over ribs and lower round. Maximum thickness (over the top round) is less than 1.5 cm.
· Intelligence. These animals are responsive and easily trained. The famous dancing cattle of Madura, for instance, are actually these banteng-cattle hybrids.
· Parasite resistance. Despite often high levels of fluke infections, the animals continue to work, reproduce, and maintain body condition.
Limitations
Maduras have been bred for one thing - speed. Some of their genetic limitations are:
· Low birth weight. The calves begin small, weighing 12-14 kg at birth, and they continue to be slow weight gainers. [Feeding trials with yearling bulls have shown daily gains of over 600 g for 180 days.
· Poor lactation. Milk production is normally less than 1.5 lifers per day and lactation ends after about 4 months. The cows often fail to produce milk. Much of this is probably caused by poor nutrition.
Research and Conservation Needs
One of the important features of the madura is that its genetic variation has been largely removed during 1,500 years of continuous breeding; with study much valuable information could be obtained that is beneficial to all bovine breeds.
The following topics need further study:
· Fertility levels
· Crossbreeding to test the effect of using a wider range of cattle breeds*
· Assessment of the hybrid's advantages over the pure banteng
· Performance under a wide range of environments.
The mithan (Bos frontalis) is believed to be a domesticated form of gaur (see chapter 6).t (However, it resembles the banteng and some authors have proposed that it is a gaur-cattle cross, others a gaur-banteng cross.) The mithan is a domestic animal indigenous to parts of India, Burma, and Bangladesh. Because of large size and the high butterfat content of its milk it is widely used to crossbreed with cattle in Bhutan. It deserves greater recognition both in Asia and elsewhere.
Appearance and Size
The mithan is a handsome animal. Bulls may occasionally exceed 1.7 m at the shoulder and weigh 1,000 kg, but the average bull is about 1.5 m tall and weighs 540 kg. Cows are shorter and weigh less.
The animal has a dorsal ridge on the crest of the shoulders, a small but pronounced dewlap, and a generally flat forehead. Mithan horns are often of unusual girth; they are straight or gently curving, and many have an enormous base that practically covers the top of the skull.
Most calves and females are brown, but adult males are generally black with white stockings on all four legs. Some, however, are light brown, white, or piebald.
In the hill ranges of Assam, where gaur are still plentiful and interbreeding between mithan and gaur frequently occurs, the mithans are massive and gaurlike. But in the Chin Hills, where gaur are scarce, the mithans have lost their bulky proportions, probably by interbreeding with cattle. With them, the high dorsal ridge on the shoulder (which lends so much to the imposing stature of the gaur) has disappeared, the horns are cowlike, and the varied coloring of the domestic cow begins to appear.
Distribution
Mithan are kept in a domesticated condition by the hill tribes of northeastern India (Mishmis, Mizo, Nagas), the Chittagong Hill tracts, and some Burmese hill ranges (Arakan and Chin Hills). It is the main domestic animal of the Nagas of Nagaland.
Status
In India feral herds totalling some 50,000 head roam the jungles of Arunachal Pradesh. Recently the Royal Government of Bhutan has established two herds by purchasing animals from Arunachal Pradesh. Bhutanese farmers have some 60,000 head of animals that are hybrids of mithan and the local breed of cattle.
The government of Bhutan is breeding mithans on government farms and distributing males to private breeders to improve the genetic base.
Habitat and Environment
The mithan is a grazing animal, but in some areas herds are allowed to browse freely in the woods; some return to the villages for protection at night, while others remain largely in the forests. The villagers keep the forest mithans nearby by providing salt, for which the animals have an insatiable craving.
Feral mithan live in the same habitat as gaurs and are said to move equally skillfully in mountainous terrain. Usually they are found at elevations from 600 to 3,000 m. However, in the Chittagong Hill tracts and the Mishmi country they descend to 300 m and lower, while in Bhutan they have been reported grazing in summer at altitudes as high as 3,300 m, for example around Thimphu.
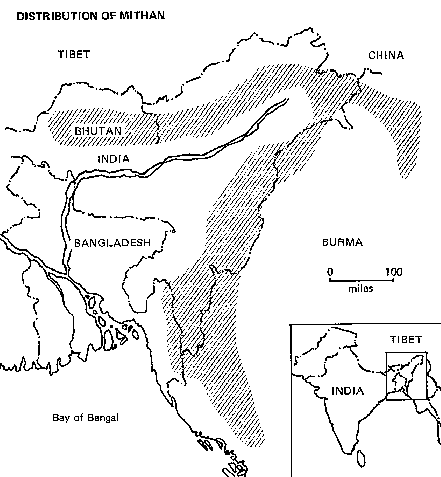
Biology
Mithans are fully fertile amongst themselves. Also, they interbreed freely with the gaur, banteng, yak, and cattle of both the taurus and zebu types. Naga owners encourage the interbreeding with gaur, regarding it as an improvement of the race. (They arrange this by placing salt licks in the forest. After gaur bulls have formed a habit of coming to the licks, mithan cows are left there and in due course mating takes place.)
The crosses between mithan and zebu are also encouraged in certain districts. Unlike most crosses between bovine species, those between mithan and cattle result in fertile male and female offspring (although some owners indicate that the F1 male is not a reliable breeder).
Behavior
This is an unusually gentle animal with a quiet disposition, as revealed in the Chin tribe's expression "gentle as a mithan." Normally even a stranger is safe to approach one; if he gives it a bit of salt, it will usually follow him about. Thus, mithans are easily managed in a regular cattlerearing operation.
Many mithans are not domesticated in the strict sense. Their herds live in a semi-tame state near jungle villages and come to settlements only in the evening to lick salt.
Uses
In some regions of northern India, mithans are used for field work and as draft animals. They are also important as a meat supply. The Bhutanese government is establishing a national dairy-mithan breeding program, which could result in a valuable dairy animal.
To many tribes of northeast India and Burma, mithans serve mainly as sacrificial animals. The Nagas use them as a kind of "currency" to pay for goods, to buy brides, and to pay penalties.
Hybrids resulting from backcrossing mithan with common cattle are also used as work animals. For at least a century, Bhutanese livestock breeders, particularly those in the eastern section, have mated mithan bulls to siri cows (Bos taurus) from India. This produces very profitable hybrid offspring that have high milk production. The milk is rich in total solids and produces exceptional yields of cheese and butter.* The male of the cross (called "jatsha") is a powerful draft animal, and the female ("jatshum") is a prized milk cow. To this day, extensive crossing continues.
Potential Advantages
The mithan is potentially an animal that can be used in difficult terrain where most domestic cattle breeds do not perform well. Mithans are superior when it comes to feeding on steep slopes and cliffs and for grazing native grass and the leaves of local fodder trees. They are also adapted to tropical and subtropical environments. And they are able to maintain themselves in small herds (6-10 head) in dense jungle.*
The mithan could prove valuable in other parts of the world, and it could be important particularly for the genetic improvement of cattle in the tropics.
Limitations
If they are disturbed, mature mithans can be temperamental. They can be difficult to hold with normal fences or chutes, because of their size. When given injections or otherwise subjected to pain, they are liable to bolt to the jungle and not return.
Research and Conservation Needs
The productivity of these animals needs to be better characterized and defined. Attention should be given to their grazing efficiency as compared with that of cattle.
The two farms the Bhutan government has established for breeding mithans provide an opportunity to gather genetic information on the species and to have experimental matings take place to establish the most suitable animal for various conditions.
The genetic relationship of mithan to gayal, gaur, and cattle needs to be clarified. Although it is believed that the mithan and gayal are the same animal, one of this report's reviewers points out that the mithan of Bhutan are strikingly different in color, body shape, and horn structure from gayals seen in zoos in Europe and India. Although the mithan is now considered a domesticated gaur, many in the past have claimed it as a gaur-cattle hybrid. Physiological research could remove lingering doubts.
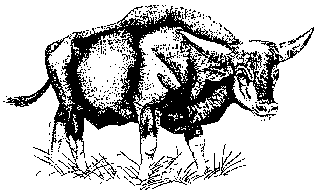
The yak (Bos grunniens), is a grazing animal that is accustomed to traveling great distances in a harsh environment. Nothing is known about when it was first domesticated, but there has probably been a close association between man and yaks ever since the first humans migrated into the high mountains of Asia.
Appearance and Size
Domesticated yaks are about the size of ordinary cattle and rarely exceed heights of 1.3 m at the shoulder. Their liveweight is generally 250-550 kg for the males and 180-350 kg for the females. They differ little from wild yaks except that they are smaller, have shorter and thinner horns, and may be rusty brown, black, silver grey, or piebald instead of black.† Often they have whitish spots on their faces.
The yak's hair is long, especially on the flanks, where it forms a shaggy fringe that often reaches the ground. This, together with an underlying layer of thick, fine wool, protects the animal from the bitter cold of its native region. The yak has an enormous tail with a brush of long hair coming from its root, which is rare in bovines.
Long extensions of the thoracic vertebrae give the yak shoulders that look like a hump, but this is different from the boneless hump in zebus. The body is long, short-legged, and compact, with particularly large forequarters because, like the bison, the yak has 14 or 15 pairs of ribs instead of the 13 of domestic cattle.
The horns spread outward and upward, and the head is held low, like that of the bison. While no hornless domestic yaks have been reported in Nepal, more than 90 percent of those found in Mongolia lack horns.
Distribution
Most yaks are found especially in the mountains and plateaus of Tibet and western China, however they occur from northern Afghanistan, Pakistan, India, and Bhutan to Mongolia, and the Soviet Union.
Status
There are more than one million domestic yaks in the world.
Habitat and Environment
In the Himalayas the domestic yak is almost always found above 2,000 m elevation. Males are sometimes brought as low as 1,700 m for mating with cattle (see chapter 6), but they do not fare well at such altitudes. In Mongolia and Buryatiya (U.S.S.R.), however, they are found as low as 1,500 m elevation.
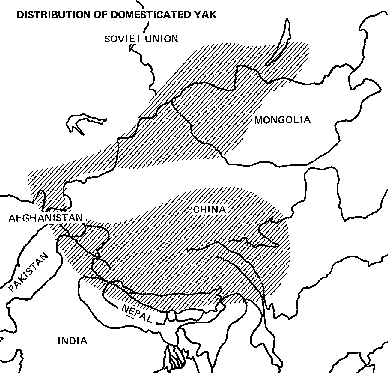
The wild yak lives in desolate mountain areas at altitudes of 4,000-6,000 m. Its habitat is the alpine tundra and the cold desert regions of the northern part of the Tibetan plateau. These barren mountain regions are remote and at higher elevations than the zones of human habitation.
Biology
Yaks look ungainly, partly because they are covered with long hair, but they are agile climbers and can maneuver narrow mountain paths. Their long hair is one adaptation to the cold climate. Other adaptations are the small, compact scrotum of the male and udder of the female, the small teats (only 2-3 cm long) and covering of hair over the udder, and the covering of short hair over the muzzle, except just around the nostrils.
In early summer yaks graze the lush grass in valley bottoms, but for much of the year they live on dry, coarse mountain grass and for long periods obtain water by eating snow.
The gestation length is 258 days - slightly shorter than that of cattle.
Behavior
Domestic yaks are docile and easily managed.
Uses
Yaks are especially useful as riding and pack animals; they can manage loads of more than 150 kg. At altitudes up to 6,000 m they may carry a pack or a person at a steady pace for days and still remain in good condition. In some regions they are the only feasible pack animal. Yak bulls (generally castrated) are also used for plowing and for threshing grain.
Occasionally the yak is slaughtered for meat. The meat is particularly important in parts of the USSR and in Mongolia, where in the cold, high-altitude environments it reportedly costs only half as much to produce as beef.
In most areas milk production is an essential aspect of yak husbandry. Yields vary considerably according to management and nutrition, but they average about 600 kg and can be more than 1,000 kg per lactation. Yak milk is golden colored. It is much richer than cow's milk; a typical analysis is: total dry matter, 17.35 percent; fat, 6.5 percent; protein, 5.3 percent; and sugars, 4.6 percent. The fat globules are much larger than those in cow's milk. People of Tibetan stock use yak butter as a food staple and as a lighting fuel.
Yak hide, though apparently not as good as that of cattle, is used for many purposes. It makes outstanding saddles. Yak hair is used for making ropes, saddle blankets (purportedly the best in the world), grainstorage bags, and tents. The fine wool that occurs beneath the long hair is used for making felt. The annual yield of adult females and males is about 750 g and 1,600 g of hair and about 350 g and 600 g of wool, respectively.
Because yaks are often found above the timberline their dung is an important fuel in many areas.
Advantages
Yaks can live and work hard in a cold climate. They are the only bovines to thrive at high altitudes. In cold, high areas they can work and produce meat and milk more efficiently and cheaply than cattle.
Limitations
Yaks are not well able to eliminate surplus heat because their heavy coat reduces their ability to sweat. In warm climates their respiration rate and body temperature increase and they become exhausted and susceptible to infections. At low altitudes in Nepal yaks die of a variety of diseases. Apparently not even yak-cattle hybrid animals (see next chapter) can successfully live below 700 m in that part of the world. This is probably due to overheating, since during the first half of this century yaks grew successfully in trials at low altitudes in Alaska and northern Canada.
Research and Conservation Needs
· Yak improvement programs are much needed. It seems probable that the genetic potential of the species is far from realized because Buddhist beliefs oppose the deliberate killing of stock. Techniques for deep freezing yak semen have been successfully developed at Regents Park Zoo, London and should prove invaluable for improving the breed.
· As a result of uncontrolled hunting the wild yak is endangered and is now restricted to remote barrens on upland plateaus and highlands in northern Tibet and Chinghai, inhospitable even to domestic yaks. The numbers of wild yak are unknown. They require total protection throughout their range and the establishment of adequately protected reserves with good pasture. To preserve their genetic purity, it is important to isolate wild yaks from domestic yaks and thus keep them pure.
· A primary need is to catalog the numbers and locations of purebred yaks. Today we have little idea of the total breeding population. Gathering such information is difficult, owing to the geographical isolation of the communities involved.
· It is also important to catalog the movement of yaks between different areas to see if enough genetic exchange occurs to prevent inbreeding of the population. Exchanges between local pastoralists were much more frequent in the past (for example, between Tibet and Nepal), but this is now more difficult because of border restrictions and because many yak
herders that used to move herds long distances have become sedentary farmers.
· Breeding strategies, herd management, product processing, and herd hygiene in Nepal, Bhutan, Sikkim, northern India, and northern Pakistan, Tibet, Mongolia, and the Soviet Union all differ greatly. It is important to get an overall perspective so that the strengths of the different systems can be judged and the weaknesses reduced.
· There are clearly different domestic yak "breeds" in terms of their appearance, and these need to be identified and compared systematically.

Yaks and cattle have the same diploid number of chromosomes (60). And in the regions where yaks are found, they are often interbred with cattle, either the humpless cattle (Bos taurus) of Tibet and Mongolia or the zebu (Bos indicus) of South Asia. As with mules, the hybrid offspring of cattle and yak surpass their parents in strength and vigor. Yakows* grow faster than their parents, and they suffer less from high temperatures than yaks. The hybrid cow reaches sexual maturity earlier and yields larger quantities of milk than the yak cow. The males, however, are sterile.
Appearance and Size
The yakow's appearance varies with the type of cattle used in the cross. But because of the phenomenon of hybrid vigor (heterosis), the hybrids are considerably bigger than the mean size of their parents. (For instance, in one test the liveweight was approximately 18 percent higher than the average weight of the parents.) The hybrids also excel in hardiness, working ability, growth rate, and milk production.
Nevertheless, their appearance and performance is closer to that of the mother than of the father. Because of this, the yakows can be "custom designed" for various altitude zones. For example, the farmer at lower altitudes may produce cattle-like yakows by breeding yak bulls to domestic cows, while farmers at higher altitudes would use the reverse cross.
Yakows have shorter hair and a much less downy undercoat than purebred yaks. They are similar in color to yaks. (In Nepal, black is most frequent, but brown shades and even white may occur.)
Distribution
Yakows are found in parts of northeastern Afghanistan, northern Pakistan, northeast and northwest India, Nepal, Sikkim, Bhutan, Tibet, western China, Mongolia, and southeastern Russia. They are found notably in the Himalayan region of Nepal, where they are bred by people of Tibetan culture and language. Sherpas, for instance, supply hybrid cows and heifers to dairy farmers throughout northeastern Nepal. Hybrids are also much bred in Ladakh (northern Pakistan) and Mongolia.
Whereas farmers in Nepal tend to use zebus to cross with yak, the inhabitants of the Tibetan plateau generally use Bos taurus cattle. People in some areas favor mating yak bulls to cows, while those in other areas prefer the reverse. But even within one village there can be a degree of specialization by different families, depending on wealth and the location of traditional grazing grounds. Generally, however, the crosses between domestic cattle bulls and yak cows are the predominant ones.
Status
The number of yak-cattle hybrids in the world is unknown.
Habitat and Environment
Like the yak, the hybrid can live in cold, barren, upland terrain, but it has the advantage of being able to adapt to lower altitudes. In winter, caravans of hybrids may come down as low as 1,600 m in some parts of Nepal, and hybrid herds (especially those resulting from crossing yak bulls with zebu cows) can spend several weeks grazing at altitudes as low as about 1,700 m in winter.
Biology
Female hybrids are fertile when mated with either parent stock. Male hybrids, however, are sterile. Although they have fully developed secondary sexual characters and show libido, their testes do not produce spermatozoa because the seminiferous tubules are poorly developed and the spermatogonia and their nuclei are degenerate.
A few normal or motile spermatozoa may be found in the semen of backcrosses of the F1 hybrids to either parent, but as a rule the spermatozoa are normal and dead.* Backcrossing is reportedly not common, however, as the progeny do not retain hybrid vigor.
Behavior
Tibetan farmers prefer the hybrids for plowing because of their docile temperament. The yak is said to be more stubborn.
Uses
The hybrids are valued as beasts of burden and draft, and are often preferred over yaks. Male hybrids are generally castrated at about 3 years of age so as to increase their strength and size.
Potential Advantages
Where pastures occur over areas of greatly varying altitude, the use of hybrid livestock is likely to be more efficient, in biological terms, than the use of yaks or cattle. By judiciously selecting cattle, yaks, or hybrids all altitudes from sea level to above 5,000 m can be utilized with best efficiency.
The hybrid's milk is intermediate in composition between that of its parents. However, hybrids yield up to 7 kg of milk per day against the yak's 3 kg. (It seems that the hybrid derived from cattle bull and female yak produces considerably more milk, with a higher fat content than that derived from yak bull x cattle female.) Also the female hybrids produce larger quantities of milk than Nepalese zebu cows on the same hill pastures. They also produce a calf each year, while under normal herd management yaks tend to produce a calf every 2 years.
Limitations
Hybrids do not tolerate extremes of altitude or cold as well as purebred yaks.
The normal gestation period for yaks is about 1 month shorter than that of cattle, and the gestation period for a hybrid is intermediate between that of the parents - hence there may be problems at calving when a female yak is sired by a bull from a large cattle breed.
Research and Conservation Needs
Research is needed to identify differences in altitude tolerance between various hybrids. This should include measurements of hemoglobin count, respiration rate, and pulse rates at different altitudes.
Studies are also needed on all production characteristics, particularly on the meat and milk production potentials.
Cataloging the breeding strategies of different areas where hybrids occur would be useful, along with a historical perspective.
Research to determine the most productive hybrids by crossing different yak and cattle "breeds" in areas with different environmental conditions could be extremely useful.
In various parts of Southeast Asia there exist little-known relatives of cattle and water buffaloes. Many of these wild animals are close to extinction, and attention is drawn to them in hope their populations will be preserved. All of them survive in tropical regions subject to environmental extremes - flooding annual rains, searing droughts, and swarming pests and parasites. These are conditions to which conventional livestock are poorly adapted.
None of Africa's bovines is threatened with extinction, and both the European and American forms of bison are being preserved by governments and individuals. In Asia, however, there has been an alarming drop in the numbers of wild water buffalo, wild yak, wild banteng, kouprey, gaur, and tamaraw in recent years.
This section describes:
· Wild banteng
· Gaur
· Kouprey
· Tamaraw
· Anoas
If not molested, many of these animals can thrive side by side with human settlements. They may therefore have an important future role in the development of the tropics.
There is little point in conservation for conservation's sake, but loss of irreplaceable resources through ignorance, greed, or thoughtlessness should be avoided. It may be that these Asian animals have little merit other than their uniqueness, but at the very least, sufficient numbers should be conserved until we have time to find out. A. J. SMITH Royal School of Veterinary Studies, Edinburgh, Scotland
Rapid human increase and relative poverty deny hope that any land can lie idle when so many would be robbed of an opportunity simply to exist. For wildlife to survive, let alone at its breathtaking best, we must think of new and better ways to justify its future, preferably by having it contribute to the welfare of those hard-pressed humans who inevitably are further disadvantaged by the creation of national parks. DAVID WESTERN New York Zoological Society, Bronx, New York, USA
There is an urgency to conserve and study nontraditional animal species, especially for use in the environmentally challenging tropical areas of the world. The philosophy plant scientists apply to exploring the potential of the plant kingdom needs to be more enthusiastically adopted for the world's animal species. G. W. M. KIRBY, Principal Animal Production Officer Department of Primary Production Darwin, Australia

The wild banteng (Bos javanicus)* are the most cowlike of all the wild bovines and are the parent stock of the 1.5 million domesticated banteng in Indonesia (see chapter 1). They have a scattered distribution throughout Southeast Asia, and three subspecies are recognized:
· The Java banteng Bos javanicus javanicus (Java and Bali)
· The Borneo banteng Bos javanicus lowi (Borneo)
· The Burma banteng Bos javanicus birmanicus (Burma, Thailand, Indochina).
Appearance and Size
Banteng have been called the most beautiful of all wild relatives of cattle. The cows are usually a vibrant reddish brown, while adult bulls are mostly blackish-brown and sometimes even blue-black (although in Burma and Indochina bulls remain golden brown like the cows, and in Thailand a few banteng have been recorded with white, deerlike spots on their brown coats). Both sexes have white "stockings" and a large white patch on their rumps.
Wild banteng are larger than their domesticated counterparts. Average-sized bulls of the Java and Burma subspecies stand 1.6 m high and weigh 635 kg, but bulls as large as 1.9 m and 825 kg have been recorded. Cows average 1.4 m in height and weigh 400 kg. The Borneo subspecies is smaller.
The horns of banteng bulls are angular, turning out and then up, with inward-pointing tips and reaching a spread of 60-75 cm. The horns of cows are short and crescent shaped. There is a patch of thick, naked skin between the horns.
Distribution
The animals are found in restricted localities scattered over an area ranging from the northeastern edge of India, through Burma, Thailand, the northern Malay Peninsula, central and southern Indochina, and the islands of Borneo, Java, and Bali.
Status
Only a few thousand wild banteng survive, and their numbers are decreasing. Most populations are endangered because their habitats are being encroached upon by the growing human population. In Sabah, Malaysia, for example, the areas where an estimated 300-550 banteng (the last remaining herds) occur are scheduled for conversion to permanent agriculture. The animals are being forced to use smaller and smaller habitats, thus increasing losses caused by malnutrition, diseases, and parasites. In addition, throughout much of the animal's range, hunting, military operations, and perhaps interbreeding with domestic cattle are further reducing the original stocks.
Habitat and Environment
The banteng's general distribution lies in the zone of deciduous monsoon forest in Southeast Asia. Habitats vary between the extremes of dry wooded parkland with large grassy plains to tropical rain forest with small clearings. The animals prefer flat or undulating terrain. In northern Kampuchea and eastern Java, they inhabit grassland savannas. In western Thailand, they live in a belt of grass and bamboo thickets along upper slopes of dry mountains.
Wild banteng inhabit sites from sea level to around 2,000 m elevation. They avoid large human settlements and plantations.
In most areas there are no pronounced hot or cold seasons, but dry seasons can be long.
Biology
Banteng prefer feeding on grass, but are fond of herbs, leaves, fruits and blossoms, as well as the sprouts of trees, brush, and young bamboo. Under favorable conditions they drink daily, preferring standing water. During droughts, they seem able to survive several days without water. In coastal areas where there are no mineral licks they can meet their need for salt by occasionally drinking seawater.
The sex ratio at birth is 140 males to 100 females, but mortality of bulls is heavy and the adult ratio is about three cows for every bull.
Calves are suckled until they are 14-16 months old.
Behavior
In undisturbed conditions, the banteng's daily activity has a more or less fixed rhythm. During daytime, the animals alternate active and resting periods of 2-3 hours each. The active periods predominantly involve feeding, drinking, and social interactions. While resting, the animals commonly ruminate.
As a reaction to heavy rains or human disturbances, the animals retreat into dense vegetation. In regions with frequent human disturbance they generally appear in the open only at night. However, where they find particularly suitable localities they become somewhat accustomed to human presence and will venture out in daylight.
Banteng live alone or in small groups of up to eight members. Males separate from their mothers at an age of 2-3 years. Sometimes female calves continue living with their mother even beyond maturity.
Cows and dominant bulls command the best pastures, and young and weaker bulls roam widely, rarely leaving the protection of the thick forest.
The banteng bull has a reputation for extraordinary savagery, but stories of its lightning attack have been exaggerated. Wildlife biologists in Indonesia have not been able to document any such attacks and have no qualms about walking in banteng habitats.
Uses
Wild banteng show important promise for improving domesticated banteng (see chapter 1) or for interbreeding with cattle (chapter 2). Almost 170 years ago Sir Stamford Raffles, founder of Singapore, noted that in Java "The degenerate domestic cows are sometimes driven into the forest to couple with the wild benteng, for the sake of improving that breed."
Tourism could be developed in areas where these magnificent animals occur.
Potential Advantages
Wild banteng are large, robust animals fully at home both in the heat and humidity of the wet season and in the hot dry season. Their genetic
endowment for such tolerance could be of significant value fin improving domestic stock.
Possible Limitations
Wild banteng may prove to have a special susceptibility to diseases of domestic animals. Blackleg (Clostridium chauvoli) and mucosal disease have caused heavy losses in banteng in European zoos.
Capture of wild banteng may prove difficult.
Research and Conservation Needs
Research on the physiology, production potential, and possible uses of wild banteng is needed.
Hunting and destruction of its habitats menace the wild banteng over all of its range. The only promising means for conservation is creation of nature reserves where hunting and forest destruction are forbidden. Conservation efforts should particularly include:
· Supporting government of Thailand efforts to conserve banteng in western Thailand and the Petchabun Range;
· Cooperating with the Burmese government in surveys to locate the best banteng habitats;
· Cooperating with the governments of Kampuchea and Laos to identify banteng habitats and establish appropriate protective measures; and
· Carrying out research in Java's nature reserves (Ujung Kulon National Park and Baluran Reserve) to ascertain the genetic distinctiveness of the banteng, its status, and its characteristics.
In Sabah and other areas of Southeast Asia there are cattle breeding projects that use imported stock exclusively. Experimental crossbreeding of local banteng with this stock should be encouraged. There are some feral and domestic banteng, as well as hybrids between wild banteng and feral cattle, that should also be tested.

The gaur (Bos gaurus) would seem to be an ideal meat-producing animal. It is a large bovine with massive muscular development, and it has already been domesticated (see mithan, chapter 3). Gaurs, which are threatened with extinction, deserve much greater attention.
Two subspecies of gaurs are recognized.
· Bos gaurus gaurus (India, Nepal)
· Bos gaurus laosiensis (Burma, Thailand, Laos, Vietnam, Kampuchea, West Malaysia).
Appearance and Size
With its huge head, massive body, and sturdy limbs, the gaur is the embodiment of vigor and strength. It is among the biggest of bovines. Bulls weigh 600-940 kg and stand 1.6-1.9 m tall at the shoulder, but a record bull of 2.2 m and 1,225 kg has been recorded. Cows are only about 10 cm shorter in height, but they are more lightly built and weigh 150 kg less.
On their shoulders gaur bulls have a striking muscular ridge that slopes down to the middle of the back, where it ends in an abrupt dip. The horns are crescent shaped, creamy yellow, and taper to a sharp point, which is usually tipped in black.
Newborns are a light golden yellow, but soon darken to coffee or reddish brown, the color of young bulls and cows. Old bulls are jet black, their bodies almost hairless. Gaurs have light colored forehead and yellowish or white stockings. Their eyes are brown but in certain lights, because of reflection, they appear blue.
Gaurs excrete an oily, aromatic sweat, unique to this species and to the mithan. It gives the animals a strong bovine smell and may be an adaptation for keeping away insects.
Distribution
Once common throughout South and Southeast Asia, gaurs now survive only in scattered remnant herds of up to 30 animals in the hill forests of India, Nepal, Burma, Thailand, Laos, Kampuchea, Vietnam, and the Malay Peninsula.
Historically, the largest concentrations have coexisted with farmers in areas of shifting cultivation. The animals adjust to disturbed land, and they also adapt to man's presence if not unduly harassed. For example, gaurs will feed in agricultural fields, along roadsides, and near occupied houses. Herds in national parks feed peacefully while tourists stand by. Gaurs in zoos also become quite tame and manageable.
Status
Populations not protected in parks and reserves are in immediate danger of extinction. Even in the remotest hill forests gaurs are harassed by hunting, exposed to the diseases of domestic cattle, and driven from their natural habitat by human invasion. Most herds outside of parks or wildlife reserves are threatened by agricultural development, hydroelectric dam projects, human settlement, or extensive logging.
In India, large populations still exist in the larger sanctuaries such as
Mudumalai and Kanha Park. In Thailand diseases carried by domestic animals, poaching, and habitat destruction have reduced total gaur numbers to fewer than 500. In Malaysia, the population is estimated to be only 400 animals. †
Habitat and Environment
Gaurs typically live on gentle, undulating terrain with natural mineral licks. They inhabit gaps in the forest, such as abandoned clearings, where they can find grasses and shrubs. In the northern portions of their range, they inhabit deciduous and semideciduous hill and mountain forests with light brush and many grassy clearings. In the lowlands they live in open bamboo jungles, grassy plains near forests, or dense forests broken by glades or open meadows. (In the forest they probably depend to some extent on the slash and burn agriculture of hill people.)
The animals appear to be adapting to increased human presence. They make use of such man-modified habitats as logged forests and fringe areas of agricultural estates that abound with grasses and early secondgrowth vegetation.
Biology
Gaurs are combination grazers and browsers. They feed on the grasses of forest openings as well as on the young leaves, fruits, twigs, and bark of shrubs and juvenile trees. In one study in Malaysia, grasses comprised 41 percent of their diet, fortes 23 percent, and woody browse 36 percent."
Gaurs develop large muscular bodies and maintain excellent condition on relatively low-quality feed. In the Malaysian study, crude protein content of grass species varied from 7.0 to 7.6 percent and phosphorus content varied from 0.11 to 0.17 percent; yet calves reached weights of 300 kg or more during their first year.
Birth and survival rates of up to 100 percent have been reported for wild gaur populations. Calves are born at any time of the year. The gestation period is 270 days, a little shorter than for banteng or domestic cattle and longer than for yak or kouprey.
Captive gaurs calve first at 2.5 years of age.
The gaur interbreeds with the mithan, and both have a diploid chromosome number of 2n = 58.
Behavior
By nature gaurs are shy and timid. As with most wild bovines, their hearing and eyesight seem comparatively poor. Their defense lies in their massive size and acute sense of smell. When a herd with juveniles is threatened by a predator, the adults form a protective circle around the young. Although individuals retreat from danger if they can, they have a unique form of threat: they approach their opponents broadside instead of head on, displaying the huge muscular body and dorsal ridge.
In common with other wild bovids, gaurs habitually visit mineral licks, which appear to be necessary to their habitat and influence the herd's movements.
Unlike water buffaloes, gaurs do not wallow. They take cover in the forest during the heat of the day and may feed at night and in the early morning during hot weather. In populated areas such as near agricultural estates, they may feed only at night to avoid people.
In the past, gaurs associated in loose herds of up to 400 animals, but today groups of only 5 to 12 animals are normally found. The herds, which are of more stable composition than those of banteng, are separated by sex for most of the year; however, during the rut stronger bulls form a series of "tending bonds" with estrous cows.†
Uses
Gaurs are thought to be interfertile with domestic cattle. If so, their attributes of size, massive muscular development, tolerance of heat and humidity, and resistance to diseases and parasites can contribute to beef production in the tropics. A gaur-cattle hybrid might also have immunity to some cattle diseases; if it retained the mild temperament of the domesticated parent, an extremely powerful beast of burden could be produced.
The gaur is a truly majestic animal. Its habit of using grassy forest clearings and salt licks makes it a likely tourist attraction in parks and reserves.
Potential Advantages
In a climate and environment where domestic cattle are susceptible to heat stress and parasite infestation, gaur thrive and maintain body condition. Further, they are able to develop large muscular bodies and maintain excellent body conditions on relatively low-quality forage by feeding on a variety of woody browse, grasses, and fortes.
Retaining its wild instincts for survival, the gaur is better able to withstand predator attacks them domestic cattle. This could be an advantage when animals graze in remote areas. Adult gaurs are strong enough to defend themselves against a predator as powerful as a tiger. In addition, they are also very protective of their young.
Limitations
Gaurs have little immunity to some cattle diseases. In many regions of India, cattle driven into the forests to graze infect gaur herds with rinderpest, foot and mouth disease, cattle plague, and other contagious diseases. Severe losses occur. Gaurs also appear very susceptible to malignant catarrhal fever.
Gaur numbers are declining throughout their range. If this trend is not reversed, it could effectively prohibit the use of gaur for domestication or crossbreeding purposes.
Gaurs are shy and excitable, making them difficult to catch, but once in captivity the animals calm down. Second generation zoo populations are easily worked and handled.
Gaurs on occasion damage cultivated crops such as young rubber trees and cassava. They require sturdy and well-kept fences.
Research and Conservation Needs
In Southeast Asia there is special need to support the efforts of the governments of Malaysia and Thailand to conserve this species and to identify important gaur populations. Similar protective measures are needed in Burma, Laos, Kampuchea, and Vietnam.
Research is needed to establish and manage new gaur herds in forest reserves and build up the gaur population in the world's zoos. Techniques have been developed to capture and release wild gaur safely.
Fertilized gaur ova have been successfully transferred into a foster Holstein cow. The cow carried the gaur fetus to a successful delivery. This could be the forerunner of an important means of rapidly expanding captive herds by transferring gaur embryos into cattle in different parts of the world.
Research is also needed on the basic physiology and production potential of gaur.
Crossbreeding experiments should be started immediately to establish the degree of interfertility between the gaur and other bovine species.
The kouprey (Bos sauveli) is the most primitive of living cattle. Its features are typical of some forms that existed in the Pleistocene era, 600,000 years ago. Discovered by Western scientists only in 1937,† the kouprey was the last large mammal to enter the biology books. It is perhaps the most primitive of living cattle and is closely allied to Bos namadicus, the wild ancestor of zebu cattle. In 1964 it was declared Cambodia's national animal. It is now perilously close to extinction, and for a decade no koupreys have been observed close enough for a positive identification by a specialist. In 1982, however, five of the animals were believed sighted in Thailand, near the border with Kampuchea. Thus there are hopes that the kouprey still exists.
Appearance and Size
The kouprey is large. Males stand 1.5-2 m high at the shoulder and may weigh 900 kg. Females are approximately three-quarters the height and weight of the males. The body is slender and long-legged. The hump from the withers to the center of the back is smaller than that of the gaur, but larger than that of the banteng. There is a well-developed dewlap from the throat to the mid-chest, which in old animals is sometimes so pendulous that it drags through the grass in front of the forelegs.
Young bulls are gray, with black on the head and dewlap. Old bulls are entirely black and cows are mouse gray or dullish light brown. As in the gaur and the banteng, the lower parts of the legs have white stockings, but kouprey stockings have a streak of dark hair down the front.
Kouprey. (P. Pfeffer)
Kouprey horns are among the longest and widest of any bovine. The tips of the adult male horns are surrounded by rough, frayed tissue caused by the animals' habit of digging in the soil with their horns. The females have slender, lyre-shaped horns that corkscrew upwards, unlike those of any other wild or domesticated cattle.
Kouprey are said to have a grace more reminiscent of deer than cattle. They move at a light trot, as fast as 32 kph.
Distribution
The kouprey's prevalence in ancient times is evidenced by prehistoric cave-paintings in Kampuchea. The animal was also a favorite of the ancient Khmers, who carved kouprey statues and featured the animal in bas-reliefs on temples, including the monuments of Angkor Wat.
Today, whatever kouprey exist are restricted to an area along both sides of the Mekong River in northern Kampuchea, the Dangrek Range, and other parts of eastern Thailand to the far south of Laos, and to the westernmost part of Vietnam. Although one or two small herds may still exist in remote pockets of Laos and Vietnam, the bulk of the population has always been centered in Kampuchea.
There are no koupreys in captivity.
Status
Because of their large size, gregarious behavior, comparatively low reproductive rate, and preference for open areas, kouprey are vulnerable to hunting. The impressive horns and other tissues are valued as trophies and as medicinals.
In 1964 it was estimated there were about 500 kouprey in Kampuchea, but by 1970 fewer than 70 were left. The subsequent fate of the species is unknown because of the years of warfare in the area. (Early in 1970, the three nominal kouprey reserves of Kampuchea were overrun by military forces.)
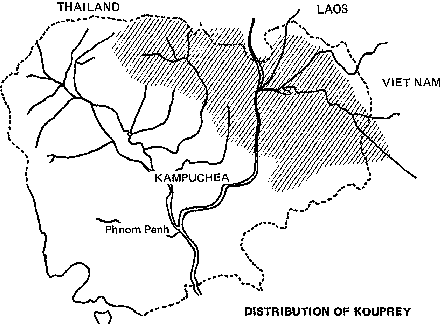
In 1975 the kouprey stocks in Laos and Thailand were estimated at only 50 and 20 animals, respectively. By now, hunting and habitat destruction probably have all but eliminated the kouprey from Thailand. However, a reported sighting of five animals that had strayed across the border from Kampuchea was made in July 1982. (By the time Thai zoologists arrived to investigate, the herd apparently had crossed back into Kampuchea.)
Habitat and Environment
Like the banteng, the kouprey inhabits open sites such as light savannas, woodland meadows, and scattered glades in the monsoon forests. It is adapted to dry country. This type of habitat is not widespread in Indochina, and the range of the kouprey is consequently limited.
Kouprey spend most of the year among low rolling hills in open areas where grazing and visibility are good. These open areas are a fire climax mosaic of dry dipterocarp savannas and patches of dense forest. Fires occur each dry season, most of them deliberately set by man. Kouprey capitalize on the young growth in burned-over areas. They feed primarily on grasses, sedges, and tree leaves. They also require salt licks and a supply of water in the dry season.
Biology
The gestation period is thought to be about 8.5 months. Cows leave the herd before parturition and return with their calves after about a month. They nurse their calves for about 6 months.
Behavior
Kouprey are said to be even more shy than banteng and gaur. They feed in erratic patterns and are always alert and nervous.
Loose herds of 20 or more have been reported. These were of mixed composition, often with more than one adult male, and were usually led by an old female. Adult bulls sometimes form bachelor herds.
Kouprey utilize some of the same feeds as wild banteng, and the two animals often live in a loose association. They graze together, and, especially after the mating season, solitary kouprey bulls often associate with banteng herds. The two species do not, however, interbreed in the wild.
Uses
The kouprey is a candidate for domestication and has important potential as breeding stock. It is a wild animal, but it may have been domesticated temporarily during the Khmer culture, 400-800 years ago.* Both Vietnam and Laos have cattle breeds that resemble kouprey, and a kouprey bull, reputed to be a domestic animal of the Stieng tribe, was exhibited in the Paris Menagerie in the mid-nineteenth century. It may be that kouprey are domestic even today in parts of Indochina.
Potential Advantages
Kouprey survival has worldwide significance. The animals could be important for studies of bovine evolution and as a genetic resource for crossbreeding to improve disease resistance and other characteristics of domesticated bovines. For instance, the kouprey is thought to be resistant to rinderpest, a killer disease of domestic cattle. Also, kouprey bulls are distinctive for their long dewlap, and they probably have more skin area for their weight than other bovines and are therefore better able to eliminate body heat. This genetic trait may help large cattle breeds survive in the tropics.
Limitations
The kouprey's potential may be moot - there may be none left in the world. It is feared that the warfare of recent years may have caused its extinction. However, if enough animals survive and can be protected, the kouprey could probably recover its numbers, as a higher calf-to-cow ratio (1:3) was reported for this species than for either banteng (1:4) or gaur (1:10).*
If wild kouprey are found, their capture is likely to be difficult.
Research and Conservation Needs
There is a long-term sequence of research needs for the kouprey, which includes the following:
. Working with the governments of Thailand, Laos, and Kampuchea to identify any remaining pockets of this species and to establish conservation programs;
· Establishing one or more captive breeding herds (because there is little chance of the animal surviving in the wild owing to the fighting and turmoil in the area);
· Studying the animal's basic physiology and production potential;
· Exploring its possible commercial utilization; and
· Investigating the kouprey's genetic relationship to zebu cattle and the transferability of genetic characters through interbreeding.
The tamaraw (Bubalus mindorensis) is related to the water buffalo, one of Asia's most important animal resources, but it has never been domesticated or studied and is threatened with extinction.
Appearance and Size
The tamaraw looks rather like a miniature water buffalo of the swamp type that is found in Southeast Asia. It reaches about I m in height and 300 kg in weight. It is much more stockily built and is blacker than the swamp buffalo. The tamaraw has white marks on the fetlocks, instead of completely white lower legs like the banteng, gaur, and kouprey. It has short (35-50 cm), very stout horns that curve out and back slightly.
Distribution
Endemic to the Philippine island of Mindoro the tamaraw is now virtually restricted to three small areas (Mount Iglet/Mount Baco, Mount Calavita, and Sablayon) in Mindoro Occidental.
Status
Because of its limited distribution, the tamaraw's future is of concern to conservationists. Until World War II, hunting was carefully regulated in its native region and no serious concern was felt for the animal's safety. Since then, however, increases in human population, lumbering, and ranching have restricted the tamaraw's habitat, and the availability of rifles and automatic weapons has seriously threatened its numbers. In 1971, research teams counted 148 head, consisting of 116 adults and 32 calves. The latest estimate is 150-200 head.
Of these last remaining animals, about 80 are located at Mount Iglit, a game refuge established in 1961 primarily to protect the tamaraw. Recently an active tamaraw conservation program has been started in a 400-hectare enclosed area on the refuge. Stockade traps have been built and tamaraw are beginning to move into them. The goal is to establish a small, semicaptive population that can be properly guarded, studied, and managed.
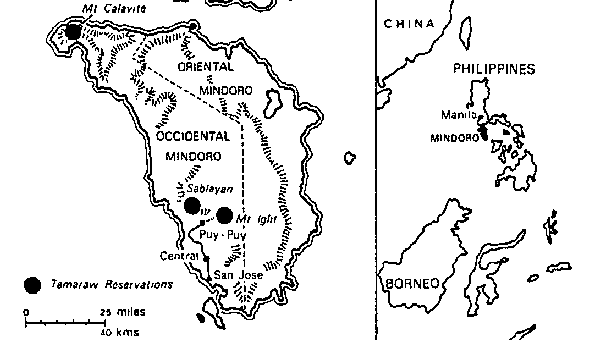
Habitat and Environment
The habitat requirements of the tamaraw appear to be fairly flexible, and present habitat use reflects human pressures rather than the animal's preferences. In the past, tamaraw were found in virtually all parts of Mindoro, from sea level to mountain tops. They occupied open grasslands or glades, but hunting pressure and habitat change have forced it to retreat into forest environments.
At present the tamaraw is found primarily in remote areas that have been partly cleared, largely by fire, so that only small pockets of trees remain among coarse grasses such as Imperata cylindrica (a widespread, unpalatable tropical weed commonly called cogon, kunai, alang alang, lalang, or blady grass), and "tahalib" (Saccharum spontaneum). The tamaraw uses the forest pockets for rest and retreat and the open land for grazing. It must have a supply of water for wallowing and drinking.
Biology
According to cattlemen, tamaraw eat both Imperata grass and tehalib but only when these are short and green. During the wet season when most forages are tall and unpalatable, the animals are known to feed on bamboo shoots.
Life expectancy is about 20 years.
Behavior
Tamaraw are reputed to be nocturnal, ferocious, and retiring. However, in earlier times they were reportedly relatively tame, diurnal animals of open areas. It was the continual hunting that made them increasingly nocturnal and secretive, and like other wild bovids they can become aggressive and dangerous when harassed or wounded. Given effective protection they doubtless would revert to placid, diurnal behavior.
Uses
The tamaraw appears to be a possible livestock animal. Its meat and hides were highly regarded by local peoples, cattlemen, and visiting hunters. Because of its close relationship to the water buffalo, it may be able to provide genetic material for improving the health and hardiness of that valuable animal resource.
Advantages
The animal is renowned for hardiness and resilience to heat, humidity, and poor forage, as well as its capability to thrive in a variety of environmental conditions.
Limitations
Tamaraws have only once produced offspring in a zoo and may prove difficult to raise in captivity. However, they have been kept in captivity so seldom that the prospects are unclear. Experiences with other wild bovids suggests that captivity and captive breeding should be no problem.
Research and Conservation Needs
The tamaraw must be protected and preserved. There is urgent need, for example, to establish more secure parks in the Philippines to rebuild the wild population to safe levels. Other conservation measures should include:
· Establishing and maintaining more reserves in natural habitats containing breeding populations, and
· Building up breeding populations in suitable zoos and livestock research centers.
Research is needed on the tamaraw's physiology, production characteristics, behavior, and genetic relationship to the water buffalo. None of these is well understood.

The lowland anoa (Bubalus depressicornis) and the mountain anoa (Bubalus quarlesi)* are small bovines that are related to the water buffalo but that are scarcely bigger than goats.
Appearance and Size
Compared with other bovines, the anoa is tiny. It stands only 0.75-1 m tall at the shoulder. The mountain anoa is smaller than its lowland counterpart. The limbs are short, the body plump, and the neck thick.
Anoa horns are short and straight, reaching a maximum length of about 380 mm; they are ringed and triangular in cross section at the base in lowland species and simple and conical in cross section in mountain species.
Although the young are thickly covered with yellowish-brown woolly hair, the mountain anoa adults tend to have curly hair, while lowland anoa have straight hair or are hairless. Adults vary from dark brown to black, with frequent blotches of white on the face, nape, throat, and lower limbs. In mountain anoas the entire lower limbs are creamy white. The underbody parts are usually light brown. Males are generally darker than females.
The hide is exceptionally thick.
Distribution
Anoas are native to dense, mature forests of Sulawesi in eastern Indonesia.
Status
Both species are abundant and well distributed on Sulawesi. Most of the northern, eastern, and southeastern peninsulas and the central area of Sulawesi are still forested and have anoa populations, and there are several large nature reserves to protect them. *
Several of the world's zoos have anoa collections (see Research Contacts).
Habitat and Environment
The climate of Sulawesi is hot, but tempered by sea winds; annual rainfall varies from 500 mm to 4,000 mm. The eastern and southeastern peninsulas have a sparse population and a scattering of subsistence agriculture.
The lowland species is found in forests and was once common along the coasts. The mountain anoa is found in primary forests at high altitudes.
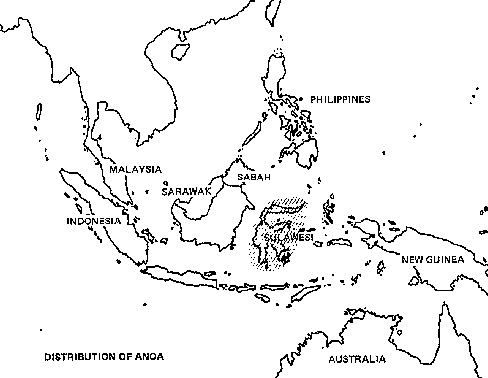
Biology
Anoas have a wide-ranging diet that includes grasses, ferns, saplings, palm, ginger, and fallen fruit, especially figs. It is of particular interest that the animals can live on a diet that contains no grass.
Anoas have a high requirement for minerals. They visit mineral-rich hot springs and salt licks, and they even drink from the sea.
The San Diego Zoo in the United States and Surabaya Zoo in Indonesia have successfully bred and reared the animals in captivity. The gestation period is 276-315 days, and there is generally only a single birth. The life expectancy is about 20-25 years.
Behavior
Anoas are shy animals, but they can be aggressive. The sideways and upwards stab of their straight, sharp horns can be dangerous.
Except when the cows are about to give birth, anoas apparently associate in pairs rather than in herds. They enjoy water and frequently wallow in mud. Their gait is a trot, but a times they make clumsy leaps. During the morning they feed alone. In the heat of the afternoon they seek refuge under shade trees.
Uses
On Sulawesi anoas are prized for their hide, horns, and meat. The flesh, especially that of calves, is tender and well flavored.
Potential Advantages
Although somewhat aggressive and pugnacious, anoas might make a suitable livestock animal. They grow and reproduce well in captivity and are adaptable and intelligent. Their small size makes them easier to handle than many other wild bovines.
Limitations
Little is known about the habits of these animals because of their wary nature, secluded environment, and restricted range. They can be tamed enough to obey commands ("lie down," for example) but they remain nervous and likely to butt strangers.
Research and Conservation Needs
Surveys are needed throughout Sulawesi to determine the distribution and taxonomy of possible races of the two species, with a view to establishing a protected-area system that will ensure the survival of the genetic diversity of this group.
Research is also needed on social organization and tameability, as well as on the anoa's biological relationship to the tamaraw and water buffalo.
One target should be to build up the zoo populations of both species.

In parts of Asia wild and feral pigs are often the most abundant source of meat. These animals are predominantly variants of the domestic pig, Sus scrofa, or of its ancestor, the Eurasian wild boar. Also contributing to the pig population are five Asian species:
· Bearded pig
· Sulawesi warty pig
· Javan warty pig
· Pigmy hog
· Babirusa
In Southeast Asia hybridization between these species and common pigs has resulted in a confusing diversity of forms and interrelationships. Because of their value, these pigs have been spread since prehistoric times by traders and migrating peoples, creating odd and unpredictable distribution patterns.
The species listed represent a gene pool of potential importance for the further development of one of man's most important sources of food.
In many areas of the world, one must start with the native animals adapted to that environment. In many cases, they are the only animals the native human population can afford to begin with. And it is amazing the increased animal production that can result from the use of better production practices with them. TONY J. CUNHA, Dean Emeritus California State Polytechnic University Pomona, California, USA
Fitting the animal to the vegetation might be a better approach than trying to fit the vegetation to the animal, especially on ranges that have been changed or degraded by man. JAMES TEER, Director Texas Parks and Wildlife Department, Austin, Texas, USA
There is now widespread realization that breed importation is not necessarily the quickest route to increased animal production. Indigenous, adapted breeds should be examined more closely and, where necessary, steps should be taken to ensure conservation of at least some of them. HELEN NEWTON TURNER Genetics Research Laboratories, CSIRO, North Ryde, New South Wales, Australia
Maximizing the animal harvest, essentially of animal protein, assumes in concept that all animals will be fully exploited in efficient and economic production systems. C.DEVENDRA Malaysian Agricultural Research and Development Institute, Selangor, Malaysia

Although it apparently has never been domesticated, the bearded pig (Sus barbatus) has a long history as an important resource in Southeast Asia. Human remains from the Niah Caves in Sarawak are accompanied by large numbers of its bones and teeth, indicating that 40,000 years ago it was the most commonly eaten large animal. Today in Sarawak and some other areas the bearded pig is still probably the most sought after source of wild meat.
Appearance and Size
Bearded pigs are large. Boars measure 1-1.6 m in length (crown to rump), up to 1 m in height, and may weigh as much as 150 kg. Sows are smaller. Adult males have small facial warts (infraocular and preocular) and a bushy tuft of hair on the cheek. Both sexes vary in color from pale red-brown to yellow-brown or black. They have elongated skulls with longer, more flexible snouts than the common pig.
Distribution
Five subspecies are recognized. They range through the Philippines (Balabac, Palawan and offshore islands, Calamianes, Luzon, Mainit, Mindanao, Jolo, Mindoro, and Cebu) to Borneo, Bangka, Sumatra, the Riau Archipelago, and the Malay Peninsula.
Status
The Borneo subspecies (Sus barbatus barbatus) is still abundant in some parts of Sabah, Sarawak (including several wildlife reserves), and
Kalimantan. It remains an important food resource for some hill tribes, although with the spread of Islam, attitudes toward pork are changing in some areas. The Malayan subspecies (Sus barbatus of) is now rare in the Malay Peninsula. It is also becoming rare in Sumatra as the lowland forests are logged and broken up by commercial interests and as the human population expands. The status of the three Philippine subspecies is currently unknown.
Habitat and Environment
The bearded pig is most commonly found in both primary and secondary evergreen forests. However, it seems to have wide adaptability, and in Sarawak bearded pigs are found in virtually all habitats from the beaches to the upland rain forests.
Biology
No biological research has yet been conducted on bearded pigs, but naturalists have made several observations. The pigs eat the seeds of trees (for example, those of species of Dipterocarpaceae and Fageceae), fallen fruits (of Moraceae, Bombacaceae, and other plant families), roots, stems of wild bananas, herbs, and probably earthworms, and along the coast they dig up and eat turtle eggs.
Births occur throughout the year in Sabah, but the peak coincides with the fruiting season of forest trees, usually August-September. The litter size is from 3 to 11 piglets.
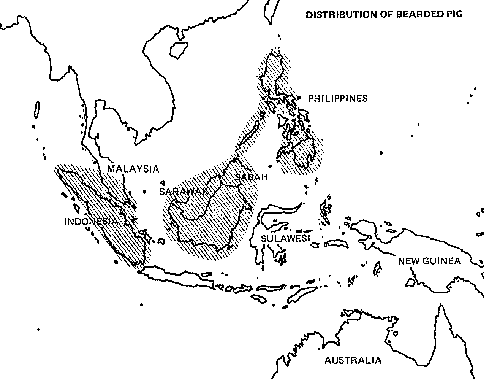
Behavior
No behavioral studies have been conducted, but observations suggest that bearded pigs are generally sedentary animals, although in some areas they congregate into large groups that may travel long distances together.
Uses
The species might be used (alone or together with other pig species) as a local source of meat, or even as the foundation of a meat industry. The bearded pig is accustomed to living in groups, which may make it suitable for husbandry or game management.
There is evidence that the bearded pig will interbreed with the common pig, producing young in which both sexes are fertile.* The progeny might have considerable hybrid vigor.
Potential Advantages
The bearded pig may have tolerance (if not actual resistance) to tropical diseases and conditions that affect the common pig. The species survives in areas of Southeast Asia where feral herds of common pigs are not common, apparently owing to their inability to cope with disease or other environmental challenges.
Limitations
No negative qualities have been reported, but the animal's biology, behavior, management, and potential uses are so far virtually unstudied.
Research and Conservation Needs
Aspects of the animal's general biology that should be investigated include:
· Chromosome type and variability, and chromosomal differences between the bearded pig and other wild and domesticated pig species
· Reproductive physiology
· Nutritional physiology
· Social behavior (both in its wild state and under controlled conditions).
To assist in the selective capture of young bearded pigs in the wild, external features that characterize the species at an early age need to be identified.
To assess the bearded pig's potential for contributing hybrid vigor, crossbreeding with other pig species should be attempted under controlled conditions.
The Sulawesi warty pig (Sus celebensis) is one of the world's few domesticated animals. It is maintained as a village or household animal in a few areas of Southeast Asia, such as on the Indonesian island of Roti. The common pigs of New Guinea and parts of the Moluccas group are hybrids between this species and the common pig.
Appearance and Size
Sulawesi warty pigs are medium sized, averaging about 60 cm high and 40-70 kg. Boars are larger than females and have longer (10 cm) tusks on both jaws. Boars have three pairs of prominent facial warts: on the snout, the cheek, and on the angle of the jaw. In sows these warts are small or absent entirely.
The animals are usually red-brown with sharply marked white and yellow undersides; older animals have a round white spot (about 3 cm in diameter) on each side of the upper cheek. Piglets have horizontal stripes on the body, which disappear as they mature.
The body is covered with scanty coarse hair, but stiff bristles, which become erect when the animal is alarmed, occur along the mid-dorsal line of the body. The longest and stiffest occur on the head and nape.
Distribution
Native to mainland Sulawesi and certain surrounding islands, the Sulawesi warty pig has been introduced to the Lesser Sunda Islands (Flores, Sumba, Roti, Semau, and Timor), the Moluccas (Malmahera and Buru), and Simaleue, a small island west of Sumatra. In some parts of the Philippines (for instance, Naujan and Mindoro Oriental) the animal either occur naturally or has been introduced.
Hybrids between the Sulawesi warty pig and the common domestic pig occur on New Guinea, Ternate, Morotai, Bacan, Amron, Seram, Kei island, Aru island, and Sulawatti.
Status
In many islands of eastern Indonesia, this species is widespread and common. In a few places it is extremely abundant, particularly at higher altitudes; in others, it has been greatly reduced by overhunting, deforestation, and expanding human settlement.
Overall, however, Sulawesi warty pig populations are declining, probably due to increased hunting and to human alterations of the animal's habitats.
Habitat and Environment
This pig inhabits varied environments, including rain forest, mountain forest, grasslands, and agricultural areas.
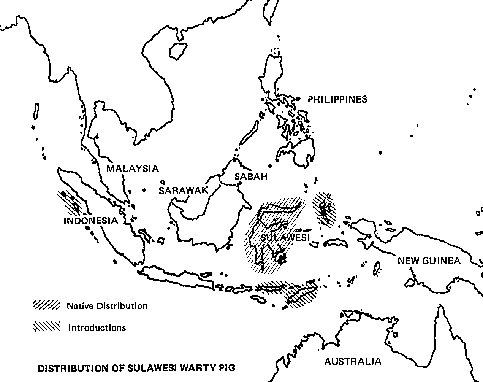
Biology
Like the common pig, the Sulawesi warty pig has broad dietary preferences. It feeds on roots, fallen fruit, leaves, and young shoots. The bulk of its food consists of vegetable materials, but it also feeds on earthworms, insects, aquatic invertebrates, rats, birds, and even carrion.
The uterus and placenta are anatomically indistinguishable from those of the common pig. The litter size is from 2 to 8 piglets, with an average of 5.
Sows can give birth throughout the year, but usually have their young in April or May. The gestation period is about 4 months.
Behavior
Breeding and farrowing occur in the forest and in open Imperata cylindrica grasslands. The pregnant female makes a nest of grasses, leaves, twigs, and branches, which she collects and places over a hole (approximately 2 m long) that she has previously dug. Here she gives birth.
Foraging is the main activity of the day and usually takes up several hours, mainly in the early morning and evening. In the wild the pigs travel in small groups, the young always traveling with an adult female. On the move, warty pigs feed and rest intermittently.
Uses
As a source of meat the Sulawesi warty pig has been recognized and exploited by local populations since prehistoric times. The presence of feral specimens far outside the pigs' natural range indicates that traders or migrants have long carried it with them on voyages, either as domestic stock or as wild specimens to be released for later capture.
The tusks, which can be carved like ivory, are a resource for local artisans. Wild specimens are suitable for sport hunting.
Potential Advantages
There is a body of unrecorded indigenous experience with this animal as a domesticate, but the information needs to be collected and appraised.
The Sulawesi warty pig may be expected to possess resistance or tolerance to the many diseases prevalent in its native habitat.
There is promising potential for hybridization between this species and domestic pigs, which might lead to the improvement of common pigs in tropical regions.
Limitations
Apart from the unwritten knowledge of the indigenous people who raise the species, very little information is available on the characteristics and management of this animal.
Research and Conservation Needs
The genetic variability within the species, as well as the karyotypic differences with common pigs and other Sus species, should be defined. Further, the hybrid vigor resulting from crossbreeding with other Sus species should be- quantified under controlled conditions. Apart from producing potentially important heat-tolerant livestock, crossbreeding may shed important light on the origins of the common pig in Asia.
The animal's nutritional requirements and reproductive biology also need study.

The Javan warty pig (Sus verrucosus) apparently has never been domesticated, but it has been a resource for hunting peoples for centuries. Now, however, the human population in its native region is predominantly Moslem, and all pigs are widely regarded as agricultural pests. Increasing numbers of them are poisoned each year.
Appearance and Size
The Javan warty pig can be up to 1.35 m long and 0.9 m tall. Mature males usually weigh between 80 and 120 kg; females are only half that weight - an unusually dramatic example of sexual dimorphism. The animal has a markedly elongated face with large warts, particularly the infraorbital wart and the smaller preorbital and mandibular warts. Body hairs can be black, red, or yellow, with black tips.
Distribution
This species is found only on the Indonesian islands of Java and Bawean. It was formerly found on Madura as well, but because of deforestation it is now thought to be extinct there. Two extant subspecies are recognized. The nominate one, Sus verrucosus verrucosus, occurs on mainland Java. The second, Sus verrucosus blouchi, a smaller animal, has recently been described on Bawean.
Status
For years it was thought that the Javan warty pig was extinct in the wild, but in October 1981 a herd was found in a small area on Mount Penanggungan in East Java. This population may prove to be substantial, and there is reason to hope that other populations can be found. Overall, however, it is clear that the Javan warty pig is endangered and declining in numbers. In many areas the animals are subjected to uncontrolled poisoning and hunting.
Five new reserves have recently been proposed to protect the animal in a variety of habitats because it is not found in appreciable numbers in any of Java's nature reserves.
The Bawean subspecies is believed to be relatively secure since the establishment of a large reserve.
A breeding colony of 12 Javan warty pigs at the Surabaya Zoo includes 5 young ones born in captivity.
Habitat and Environment
Habitats of the Javan warty pig appear to be confined to elevations below about 800 m. The animals prefer relatively large expanses of grassland or secondary vegetation, where human population is sparse.
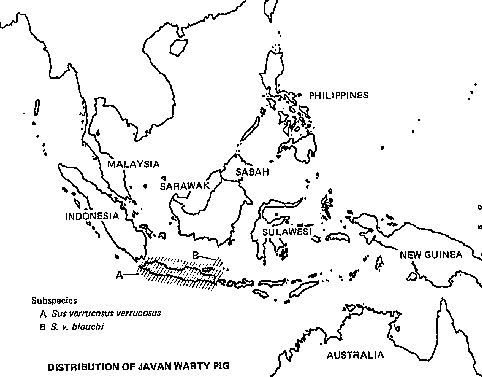
Biology
The piglets can be born throughout the year; in one study of 8 sows the litter size ranged from 2 to 8, with a mean of 5.
The (diploid) chromosome number has been found to be 38, the same as that of the common pig. Hybridization between these species is thought to occur in the wild, but apparently not with enough frequency to contaminate the Javan warty pig gene pool.
Behavior
Unreported.
Uses
These are omnivorous, adaptable animals that might make useful domesticates.
Potential Advantages
The small size of the sows could make this species particularly appropriate for households or smallholder farms. The animal's genetic "distance" from the domestic pig may make it a useful generator of hybrid vigor in crossbreeds. It could have particular value because its meat is much leaner than pork from the common pig.
Limitations
The numbers in the wild are now too few to permit any cropping.
Research and Conservation Needs
Efforts should be directed towards locating and breeding up the Javan warty pig populations that still exist. An immediate need is to isolate pure populations in reserves because it is essential to protect the animal from crossbreeding with other species. In addition, current specimens in zoos should be cataloged and a breeding program established. Other captive breeding colonies should also be started.
It is important to determine the genetic differences between the Javan warty pig and the common pig, as well as the levels of existing hybridization between them. It is also necessary to determine what variation in karyotype may exist within the populations.
Basic ecological and behavioral studies are needed, as well as studies of the dietary requirements, growth rates, and nutritional physiology of captive specimens. The reproductive biology of the animal should be studied, and the possibility of crossbreeding it with other Sus species should be examined under controlled conditions.
The Javan warty pig's potential for local meat production should be evaluated. The benefit to rural people of converting an agricultural pest into a profitable source of income should be explored within the local religious context.

The pigmy hog (Sus salvanius), a shy and very small pig of northeastern India, is close to extinction because of hunters and the destruction of its habitat.
Appearance and Size
The pigmy hog is only about 60 cm long, with a shoulder height of about 25 cm and a body weight of less than 10 kg. The hair is medium brown on the sides, darkening to blackish brown along the mid-dorsal line. A facial band of short, dark hair extends from the bridge of the nose to below the eye. The tiny tail is only 3 cm long.
Distribution
This animal was once widely found along the southern foothills of the Himalayas; today, it is definitely known to occur in only one area, the Manas National Park in Assam.
Status
Pigmy hogs are seriously endangered; in the wild they are close to extinction. Captive animals are now reduced to a single male in the Assam State Zoo and four male siblings in the Zurich Zoo.
Habitat and Environment
The few remaining pigmy hogs mainly inhabit tall-grass savannas, but it seems likely that they could adapt to other environments.
Pigmy hog. (W.L.R. Oliver)
Biology
The animal is omnivorous and consumes roots, tubers, grass, leaves, insects, earthworms, eggs, and carrion. While foraging, it undoubtedly consumes large quantities of earth as well.
The chromosome number, 38, is the same as that of the common pig. The karyotype is similar to that the common pig, but small, significant differences have been demonstrated using chromosome banding techniques.*
The age at puberty, time of weaning, length of gestation and estrous cycle, and season of breeding are not yet known with certainty. However, the animal is known to have a single well-defined birth peak (April/May) that coincides with the onset of the rainy season when the food supply increases. The uterus and placenta are anatomically similar to those of the common pig. The litter size varies from 2 to 6. It is not known if the animal can be crossbred with the common pig.
Behavior
The animals are shy, but can be tamed. They tend to forage and run in groups. Nest building is carried out by both males and females and is not restricted to pre-farrowing periods.
Uses
There is no evidence that the pigmy hog has ever been domesticated; it has, however, been extensively hunted and trapped, and recently it was still being sold for human consumption. There would probably be no inherent difficulty in maintaining this species in husbandry.
Potential Advantages
The pigmy hog's small size may make it useful in studies of the physiology of pigs and like mammals. In particular, a study of the uterine capacity may contribute to our understanding of the maternal factors that influence the number and size of mammalian young at birth.
It is unknown if the animal carries genetic resistance to diseases of the domestic pig, but given its habitat, such resistance seems likely.
Limitations
The small numbers of surviving pigmy hogs obviate any consideration of its use in husbandry at the present time. Moreover, its nervous temperament might restrict its potential as a domesticate.
Research and Conservation Needs
It is essential to ensure the survival of this animal. Efforts should be directed towards locating and breeding up the populations that still exist. If its habitat could be protected by preventing the annual dry-season burning of grasses, it is possible that substantial populations could be established. Attempts should also be made to acquire two or more females as mates for the male pigmy hogs at the Assam and Zurich zoos.
There is also an urgent need to ensure that the animals breed successfully in captivity, and the wealth of international expertise in pig reproduction should be applied to that end. For example, the reproductive biology of the animal could be studied by swine experts; the age at puberty, the length of the estrous cycle, the season of breeding (and whether it is influenced by light or temperature), the length of gestation, and time of weaning all need to be determined.
The animal is an omnivore, but the physiology of its digestion has not been studied. Thus, its nutritive requirements are not well enough known to ensure its survival in captivity or reserves.
Experiments should be made to determine if pigmy hog embryos can be brought to term in the uterus of the common pig. If so, such embryo transfers could be used to distribute embryos and set up new herds, lessening the risk of this species" extinction.
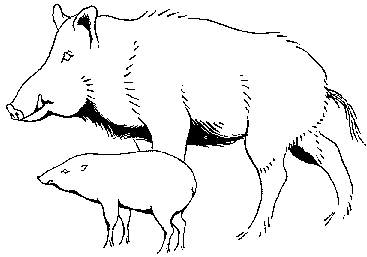
The babirusa (Babyrousa babyrussa) is a piglike animal whose closest relative appears to be an ancestral animal that lived in Europe 35 million years ago. It is easily tamed, and in its native area there is an ancient tradition of raising young babirusa for meat and for the males' unique tusks. The animal appears to reproduce well in captivity, and with good management techniques it might gain wide use in the tropics.
Appearance and Size
Male babirusa can be up to 110 cm long and 80 cm tall and weigh up to 100 kg; females are smaller. The male has large upper canines that grow upwards, piercing right through the flesh of the snout and curving back and downwards towards the forehead without ever entering the mouth. The female may sometimes have small upper canines projecting through the skin of the upper lip.
The animal is more slender than a pig of similar size. It has a gray or brown-grey skin color, although one subspecies, Babyrousa babyrussa babyrussa, has light body hair that is fawn colored or black.
Distribution
The babirusa is unique to a few islands of eastern Indonesia: north, central, and southeast Sulawesi, the Togian Islands, and the Sula (Taliabu and Sulabesi) and Buru Islands. On Sula and Buru it is probably not native but was introduced in prehistoric times.
Status
On Sulawesi the babirusa remains abundant, despite hunting and the widespread clearing of the forest. Nevertheless, disturbances created by wood and rattan collectors, hunters, loggers, and farmers threaten the babirusa's survival. Throughout the rest of its range, it is also vulnerable to extinction.
Biologists are particularly concerned about some of the babirusa subspecies. The one from the north of mainland Sulawesi, Babyrousa babyrussa celebensis, is still relatively abundant in places and is probably in no immediate danger. The Togian Islands' subspecies, Babyrousa babyrussa togeanensis, is abundant in small islands but is threatened by Indonesia's settlement programs and deforestation. The Buru and Sula Islands subspecies, Babyrousa babyrussa babyrussa, may already be extinct; there have been no confirmed sightings in recent years.
In 1981, there were 26 male and 27 female babirusa held in six zoo collections; 22 of these animals are in the Surabaya Zoo in Indonesia, where they breed well. Most, if not all, are believed to belong to the mainland Sulawesi race.
Habitat and Environment
The animals mainly inhabit moist forests at low altitudes.
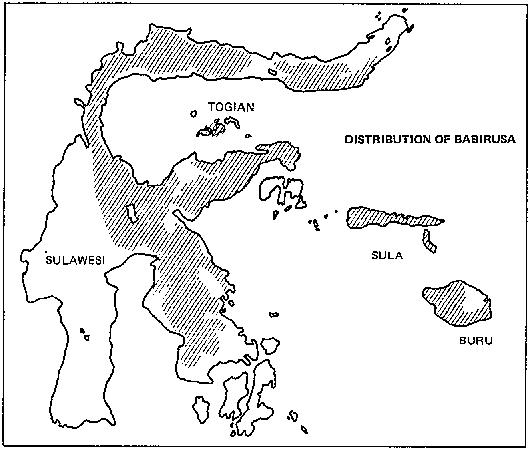
Biology
Precise details of babirusa biology are unknown, but the stomach has an extra sac, suggesting that the animals may have some ability to break down cellulose. Indeed, they browse leaves, a behavior more often found in a deer than in a pig, and have been referred to as "ruminant pigs." Babirusa also live on roots, berries, and grubs, making them true omnivores. Compared to other pigs, they do little rooting, and in captivity their enclosures remain quite grassy.
Babirusa are said to be sexually mature at 5-10 months, but this depends on nutrition. Gestation length is about 158 days. One or two young are produced although there are some reports of litters of three.
One specimen was kept for 24 years in captivity.
Behavior
The babirusa is a social animal that moves in groups. A retiring native of the dense jungle, it is a fast runner and swims readily. Mating behavior in captivity generally resembles that of domestic pig. Evidence from zoo animals suggests that the male must be removed from the young at birth but that by the time they are a month old the young are safe from paternal attack.
Uses
When captured young or reared in captivity, the babirusa is easily tamed. It has potential as a domesticated species, and with appropriate management may provide a useful source of meat. The meat is tasty and of good quality.
Because of its unique tusks, the skull of the male finds ready markets. This could provide additional income to farmers raising babirusa for meat. The ivory of the tusks could also be a resource for local artisans.
Potential Advantages
As noted, the anatomy of the stomach suggests that the babirusa may be able to make more efficient use of fibrous foodstuffs than other pigs.
Limitations
The babirusa produces only one or two young after a gestation period of just over five months; it may therefore take considerable time to build up herds.
Although the babirusa is easily tamed, it is not known whether it can be husbanded in large groups. Also, present lack of knowledge of the animal's nutrition may restrict its husbandry.
Research and Conservation Needs
The number of animals in the wild is decreasing, and attempts should be made to determine their exact status. Particular attention should be paid to the subspecies Babyrousa babyrussa togeanensis and Babyrousa babyrussa babyrussa. Captive breeding programs for these subspecies should be started.
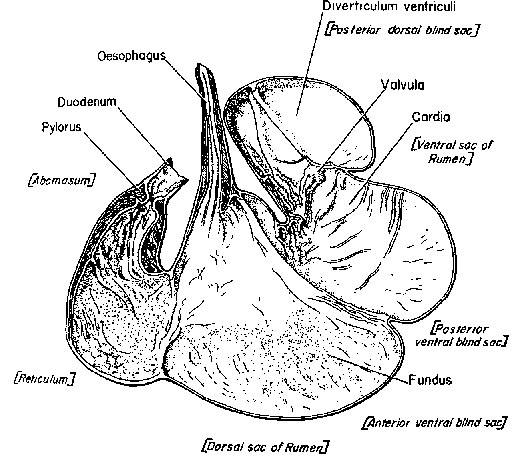
Wild populations should be maintained in several regions to ensure that the species" genetic base is retained. Advantage could be taken of the large number of islands in eastern Indonesia to establish reserves for the different babirusa subspecies.
Currently, little is known about the biology of the animal in the wild— its diet, social behavior, or reproductive performance are almost unstudied. In view of the relatively small number of babirusa in zoos and the scarcity of information about the animal's growth rate and general biology, coordinated studies between zoos could provide much basic information. It has been suggested that a studbook be initiated for this species.
It is clear from chromosome analysis that this pig differs markedly in its karyotype from that of other pigs. However, more anatomical and biochemical knowledge is needed.
Basic parameters of the animal's reproductive physiology are not known. Questions to be answered include:
· Can the babirusa be induced to reproduce twice per year?
· Can babirusa embryos be developed to term in the uterus of the domestic pig? If so, can the number of babirusas be rapidly multiplied in this way?
· Does the babirusa genotypic and gestation-length difference with the common pig prevent the two from successfully crossbreeding? And if crossbreeding can be achieved, will the progeny be fertile?
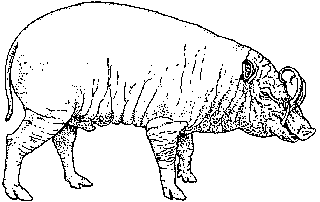
The Asian animals described in this report are a natural resource whose potential is barely glimpsed. They are virtually unknown to established livestock interests and there has been little thought to developing them as livestock. As a result, the research and trials that could lead to further use of these animals is scattered, small scale, and conducted outside the mainstream of livestock science, mainly by wildlife conservationists.
Yet these animals seem worth considering for use in husbandry in many tropical regions, especially where poor grazing and harsh environments limit the performance of conventional livestock. There is a pressing need for research to develop these species and to explore their potential. Only through rapid action can many of them be kept available for study and possible inclusion in agriculture.
One suggestion is that funds be provided to establish an Asian native-livestock research-support team. Its primary purpose would be to assist in the funding of research projects and to coordinate research being done in various Asian countries. The team's goal would be to establish a network of animal scientists based at institutions in Asia and elsewhere to work cooperatively on the region's little-known bovines and pigs. In this manner, relatively small sums of money could be used to stimulate and coordinate Asia-wide research on indigenous animals.*
Basic Research
The time has come for basic studies on each of the 15 animals discussed in this book. For each species, the following areas should be investigated:
· Physiology
· Reproductive requirements and fertility
· Nutritional requirements (such as food preferences, feeding strategies, and food utilization)
· Growth
· Adaptability and environmental tolerance (for instance, water balance, sweat rate, and shade-seeking habits, as well as temperature, pulse, and respiration rates, both under normal conditions and heat stress)
· Diseases
. Management (including stocking rates and other production characteristics
· Genetic selection for calm temperament, quick growth, and other desirable qualities
· Crossbreeding to take advantage of hybrid vigor
· Social structure (for example population density and distribution, social organization, leadership, herding potential, aggressiveness of bulls)
· Habitat characteristics (behavior-habitat interactions and herd dynamics, for instance).
Studies are also needed on better utilization of products, such as milk, meat, hides, horn, and hair, from these animals.
Experimental transfer of fertilized eggs of all of these animals into domestic species should be explored with a view to establishing herds in secure and disease-free areas.
Efforts specific to domesticated and wild species, and to hybrids of these species, are discussed below. The report concludes with brief discussions of a few promising species not taken up in the body of the text.
Domesticated Species Improving Performance
Little data is available on the performance of the domesticated animals discussed in this report. Yet the main value of these species lies in the new and superior genes and gene combinations that can be adapted to animal production in the tropics.
Studies need to be conducted with the indigenous species and strains in their native countries to determine if they will respond to improved conditions with improved performance. These projects could provide opportunities for geneticists and animal breeders to improve livestock production while conducting challenging basic research. It would be helpful to establish studbooks of captive specimens and to select and breed for desirable genetic qualities.
Genetic improvement must be directed towards remedying production deficiencies. Methods include selection of superior breeding animals, crossbreeding, and combinations of selection and crossbreeding. Different genetic improvement techniques will be required for each species. Progress should be regularly monitored against the unselected animals and the domestic livestock breeds.
New Introductions
Tropical countries should import breeding stock to evaluate the performance of some of these Asian animals in the local environment. Strict quarantine procedures must, of course, be observed.
Special emphasis should be placed on the domesticated banteng of eastern Indonesia. These animals are already an important source of human food and they may have considerable potential for increasing production elsewhere.
Wild Animals
Wild animals contribute extensively to the welfare of people and economies of developing countries. In many areas they supply much of the animal protein consumed. But the contributions they make are largely unrecorded, and local officials and aid agencies seldom regard wildlife as an economic resource. As a result, few developing countries have analyzed how their people use indigenous animals for subsistence, and few nutritional studies recognize "bushmeat," although large numbers of people eat it.
Wildlife's contribution justifies more detailed and precise evaluations. In many tropical regions few people even know what species are present, let alone in what numbers. Ecologists and animal scientists should be employed to identify and collect data on the fauna of the tropical forests and how it is used by local people. Their work will provide baseline information on the species present and on the potential utility of the undisturbed natural ecosystems.
Initial Needs
All wildlife utilization must be based on a thorough knowledge of the resource, its numbers, the animals' condition, annual recruitment in the
various populations, and the seasonal movements of the herds. In addition, it is important to understand the interactions of the species with its habitat.
Thus, the immediate need is for action to assure the preservation of populations of these animals until research studies can be conducted.
In this regard, priority actions include the following:
· Establishing adequate preserves
· Providing total protection for remaining animals
· Preventing poaching.
With few exceptions, the wild species described in this report are under the pressures of agricultural and human expansion. Without efforts to preserve their habitats it will be difficult for these species to survive.
Developing Wildlife Resources
Governments cannot stop hungry people from hunting game, but they can use this impetus to help protect some indigenous animals as well as forest and woodland habitats. Fostering the production and supply of wild animals such as those described in this book could open up a new: source of meat and other products, particularly for subsistence farmers in remote areas. In the last analysis, this may be the only way of protecting and maintaining these animals and developing local awareness of their value.
International economic development organizations have traditionally shown little interest in wildlife or wildlife habitats. Yet maintaining these animals and their habitats is crucial to the continuing supply of many indigenous food animals, the most important of which are often the smaller ones that flourish in secondary growth. And the forests that support wildlife also protect some of the huge investments that AID, the World Bank, and other other agencies have made to help developing countries. The forest is the sponge that absorbs the water from tropical rains. Without trees covering the watersheds, heavy rains cause rushing water that erodes the land, despoils highways, silts up dams and reservoirs, knocks out bridges, and inundates towns, villages, and farm fields.
In principle, the farming of tropical wildlife could become a force in preventing clear-felling of the tropical forests. Rational utilization of tropical animals offers productive alternative use of the land. In some cases, the farming of indigenous animals can fit smoothly into tropical habitats and into traditional village life, because the livestock and its needs are usually familiar to local people and are often ingrained in their traditions.( For successful examples see companion reports nos. 44 and 45, Butterfly Farming in Papua New Guinea and Crocodiles as a Resource for the Tropics.
Hybrid Animals)
Hybridization may offer new prospects for using underexploited tropical animal resources. This is a speculative notion, but it deserves serious attention.
Crossbreeding between the animals mentioned in this report holds exciting possibilities for expanding livestock options in the tropics. Some of the crosses, known or envisaged, are:
· gaur x cattle
· banteng x cattle
· kouprey x cattle
· yak x cattle
· yak x banteng
· anoa x water buffalo
· tamaraw x water buffalo
· domestic pig x warty pigs
· warty pigs x bearded pig
Care must be taken, however, to guard against the dilution of wild genes by the escape of hybrid or interbreeding domestic species, or the establishment of feral hybrid populations.
International Exchange of Pig Germ Plasm
Present veterinary restrictions effectively prevent the movement of pigs between most countries. Relatively few species of wild pigs are therefore maintained in zoos outside their countries of origin. This stifles the promising potential for the species described in Part III, as well as for captive-breeding safeguards and research on the endangered species. It is important that attempts be made to identify channels for the future establishment of viable captive stocks both within Asia and elsewhere. Transfer of embryos should be verified as a means of permitting disease-free movement across frontiers.
Species Not Covered in this Report
This project, which began as a short report on banteng, quickly developed momentum as it brought in suggestion after suggestion of further species. Through pressures of time and budget, it was not possible to take up all of these interesting animals. Some of the omitted ones are listed below.
Takin (Budorcas taxicolor).* A heavily built, Southeast Asian hoofed mammal of the family Bovidae, the takin lives in small herds in the mountains, often above timberline. Though robust and short-legged, it can move about quickly and easily over difficult slopes. It stands up to about 1 m at the shoulder and has a shaggy yellowish- to blackish-brown coat. Both sexes have heavy horns that turn outward from the center of the forehead and then curve up and backward.
Goral (Naemorhedus goral).* This small Asian goatlike bovine has slightly backward curving cylindrical horns and a coarse brownish-grey coat. It is related to the chamois and serow (see below) but is distinguished from them by peculiarities in skull form, as well as by smaller size, shorter horns, and the absence of face glands. Gorals are native to a vast area, ranging from the Himalayas to eastern Siberia.
Serow (Capricorhis sumatrensis). The serow has been called a "goatantelope." Found in Sumatra, the Malay Peninsula, Vietnam, Thailand, Burma, the eastern Himalayas, and China, it lives on forest-clad slopes, such as the mountains of southern China that are the home of the giant panda. Serows have coarse black hair, long pointed ears, a distinct mane, and short, backward pointing horns.
Native species of goats are also valuable genetic resources of Asia and examples include ibex (Capra ibex), markhor (Capra falconeri), and bharal (Pseudois nayaur). There are also various species of sheep, including the various races of argalis (Ovis ammon), which form small herds in the mountains, often above timberline. Although robust and short-legged, these animals can move about quickly and easily over difficult slopes. Soviet animal breeders apparently are exploring some of the possibilities of using argalis in animal husbandry. They are crossing domestic sheep with arkhar (Ovis ammon karelini) to produce a commercially viable hybrid.
The marketable size of lamb might be increased appreciably to provide larger, leaner cuts of meat by crossing domestic sheep with the Marco Polo sheep (Ovis ammon polii), which is as big as deer and has a mature body weight of 400-500 pounds. Marco Polo sheep meat is reported to have none of the "mutton" favor of domestic sheep. Thus, crosses might enhance consumer acceptance of sheep meat.
Asia also has several species of deer that could have been included.§ The musk deer (Moschus moschiferus) is now domesticated in China for musk production and is a resource with a promising future. Also, sambar deer (Cervus unicolor), Asia's largest deer, may prove useful in husbandry. Several deer species are being farmed in New Zealand and ruse deer (Cervus rusa), which are native to South and Southeast Asia, are being farmed in Mauritius, Australia, and Papua New Guinea for meat and medicinal by-products. Two small, goat-sized Asian deer, the hog deer (Cervus porcinus) and the barking deer (Muntiacus muntjak), may also be worth considering.

General
Bohlken, H. 1961. Haustiere und Zoologische Systematik. Zeitschrift fur Tierzuchtung und Zuchtungsbiologie76:107-113.
Boonsong Lekagul, and J. A. McNeely. 1977. Mammals of Thailand. Association for the Conservation of Wildlife, Bangkok, Thailand. 758 pp.
Corbet, G. B., and J. E. Hill. 1980. A World List of Mammalian Species. British Museum of Natural History, London, England.
Groves, C. P. 1981. Ancestors for the Pigs, Taxonomy and Phylogeny of the Genus Sus. Department of Prehistory Technical Bulletin No. 3. Australian National University Press, Canberra, Australia. 96 pp.
Gray, A. P. 1972. Mammalian Hybrids: A Check-List with Bibliography. Commonwealth Technical Communication No. 10 (Rev.). Bureau of Animal Breeding and Genetics, Edinburgh, Scotland.
Honacki, J. H., K. E. Kinman, and J. W. Koeppl. 1982. Mammal Species of the World. Allen Press, Lawrence, Kansas, USA. 694 pp.
Mochi, U. and T. D. Carter. 1953. Hoofed Mammals of the World. Charles Scribner's Sons, New York.
Payne, W. J. A. 1970. Cattle Production in the Tropics. Longman, London, England.
Robbins, C. T. In Press. Wildlife Feeding and Nutrition. Academic Press, New York, USA.
Walker, E. P. 1975. Mammals of the World. The Johns Hopkins University Press, Baltimore, Maryland, USA, and London, England.
Wharton, C. H. 1969. Man, fire and wild cattle in Southeast Asia. Tall Timbers Fire Ecology Conference 8: 107-167.
Domesticated and Wild Banteng
Appleton, D. C., G. Dryden, and A. C. Kondos. 1976. A comparison of the digestive efficiency of the water buffalo and Brahman and Banteng cattle. In Proceedings of the Australian Society of Animal Production 11.
Budiarso, I. T., and S. Hardjosworo. 1976. Jembrana disease in Bali cattle. Australian Veterinary Journal 52(2):97.
Copland, R. S. 1974. Observations on banteng cattle in Sabah. Tropical Animal Health and Production 6(2):89-94.
Darmadja, D., and P. Sutedja. 1975. Gestation period and calving interval in Bali cattle. Majalah Ilmiah Universitas Udayana 9:61 -65.
Darmadja, D., and P. Sutedja. 1976. Masa kebuntingan den interval beranak padas sapi Bali. In Proceedings, Seminar Reproduksi den Performance Sapi Bali, April 5-6, 1976. Dinas Peternakan Daerah Ternak Institute, Bali, Indonesia.
Devendra, C., T. Lee Kok Choc, and M. Pathmasingam. 1973. The productivity of Bali cattle in Malaysia. Malaysian Agricultural Journal 49(2):183-197.
Holmes, J. H. C., J. H. Schottler, and T. F. Leche. 1977. Southeast Asian cattle, buffalo, and beef in Papua New Guinea. In Agriculture in the Tropics, B. A. C. Enyi and T. H. Varghese, eds. University of Papua New Guinea, Port Moresby, Papua New Guinea.
Hoogerwerf, A. 1970. Udjung Kulan, The Land of the Last Javan Rhinoceros. E. J. Brill, Leiden, The Netherlands.
Hooijer, D. A. 1956. The valid name of the banteng Bibos javanicus d'Alton. Zoologische Mededelingenuitgegeven door het Rijksmuseum van Natuurlijke Historie te Leiden 34:223-226.
Jellinek, P. J., J. Avenell, A. Thahar, and P. Sitorus. 1980. Infertility associated with crossbreeding of Bali cattle. In Proceedings, 2nd Ruminant Seminar, Ciawi, Indonesia. 79 pp.
Jenkinson, D. M., and T. Nay. 1975. The sweat glands and hair follicles of different species of Bovidae. Australian Journal of Biological Science 28:55-68.
Kirby, G. W. M. 1979. Bali cattle in Australia. World Animal Review 31 :24-29.
Mason, 1. L. In Press. Evolution of Domesticated Animals. Longman, London, England.
Meijer, W. C. P. 1962. Das Balirind (Balinese Cattle). Die Neue Brehm-Bucherei, No. 303, A. Ziemsen Verlag, Wittenberg Lutherstadt, Federal Republic of Germany. (Translated from the Dutch by E. Mohr.)
Moran, J. B. 1973a. Liveweight changes of Brahman cross, Shorthorn, Banteng and buffalo steers grazing improved pastures at Darwin, Northern Territory. Journal of the Australian Institute of Agricultural Science 39:68-9.
Moran, J. B. 1973b. Heat tolerance of Brahman cross, buffalo Banteng and Shorthorn steers during exposure to sun and as a result of exercise. Australian Journal of Agricultural Research 24:775-782.
Moran, J. B. 1978. Digestibility and water metabolism in Brahman cross, buffalo, Banteng and Shorthorn steers fed roughage diets. Journal of the Australian Institute of Agricultural Science 44: 57-59.
Moran, J. B. 1980. The prediction of chemical content of the whole carcass from the physically-dissected rib in cattle and buffaloes. Proceedings of the Australian Society of Animal Production 13:503.
Moran, J. B. 1981. Beef and buffalo production by smallholders in Indonesia. World Review of Animal Production 17:55-64.
Moran, J. B. 1982. The performance of Indonesian beef breeds under traditional and improved management systems. Pp. 347-351 in Animal Production and Health in the Tropics, M. R. Jainudeen and A. R. Omar, eds. Universiti Pertanian Malaysia, Serdang, Selangor, Malaysia.
Moran, J. B., B. W. Norton, and J. V. Nolan. 1979. The intake, digestibility and utilization of a low quality roughage by Brahman cross, buffalo, Banteng and Shorthorn steers. Australian Journal of Agricultural Research 30:333-340.
Namikawa, T., and W. Widodo. 1978. Electrophoretic variations of hemoglobin and serum albumin in the Indonesian cattle including Bali cattle (Bos banteng). Japanese Journal of Zootechnical Science 49(11):817-827.
Nitis, 1. M., K. Rika, M. Supardjata, K. D. Nurbudhi, and L. R. Humphreys. 1976. Productivity of improved pastures grazed by Bali cattle under coconuts: a preliminary report. Universitas Udayana, Denpasar, Bali, Indonesia. 18 pp.
Nitis, 1. M., and K. Rika. 1978. Bali cattle grazing improved pasture under coconuts. 1. Effects of stocking rate on steer performance and coconut yield. In Seminar on Integration of Animals with Plantation Crops, April 13-15. Preprint No. 10. Penang, Indonesia. 8 pp
Nitis, 1. M. 1979a. Effects of replacing 30 percent of the green roughage with concentrate on the performance of Bali steer. Progress report to the International Development Research Centre, Ottawa, Canada. Centre file:3-P77-0087. 25 pp.
Nitis, 1. M. 1979b. Performance of Bali cattle fed grass supplemented with stylosanthes. Progress report to the International Foundation for Science, Sweden. Project No. RR 76. 15 pp.
Nitis, 1. M., K. Lana, 1. B. Sudana, N. Sutji, and 1. C. N. Sarka. I980. Persediaan den kebutuhan hijauan ternak di Bali (Roughage supply and requirement of livestock in
Bali). Fakultas Kedokteran Hewan den Peternakan, Universitas Udayana, Denpasar, Bali, Indonesia. 218 pp. (Report series mimeograph.)
Norton, B. W., J. B. Moran, and J. V. Nolan. 1979. Nitrogen metabolism in Brahman cross, buffalo, Banteng and Shorthorn steers fed on low quality roughage. Australian Journal of Agricultural Research 30:341.
Nursamad, M. 1972. Berat lahir den petumbuhan anak sapi Bali selama 4 bulan di kecamatan Watang Pulu, kabupaten Sidenreng, Sulawesi Selatan. Laporan, Fakultas Peternakan Universitas Hasanuddin yang berafinasi dengan Fakultas Peternakan Institut Pertanian Bogor, Bogor, Indonesia.
Pastika, M., and D. Darmadja. 1976. Performance reproduksi sapi Bali. In Proceedings, Seminar Reproduksi den Performance Sapi Bali, April 5-6, 1976. Dinas Peternakan Daerah Ternak Institut, Bali, lndonesia.
Payne, W. J. A., and D. H. L. Rollinson. 1973. Bali cattle. World Animal Review 7: 13-21.
Pulungan, H., and K. Ma'sum. 1977. Performance sapi Bali den hasil persilangannya dengan sapi Hereford den Shorthorn. Laporan Khusus Lembaga Penelitian Peternakan, Cabang Grati 1977 (Abstract).
Robinson, D. W. 1977. Livestock in Indonesia. Research Report No. 1. Centre for Animal Research and Development, Bogor, Indonesia.
Siebert, B. D., and W. V. MacFarlane. 1969. Body water content and water turnover of tropical Bos taurus, Bos indicus, Bibos banteng, and Bos bubalus bubalis. Australian Journal of Agricultural Research 20:613-622.
Siregar, M. G., and 1. M. Suparjata. 1975. A half century existence of Bali cattle in South Sulawesi. Majalah llmiah Universitas Udayana 9: 13-21.
Subandriyo, P. Sitorus, M. Zulbardi, and A. Roesyat. 1979. Performance of Bali cattle, as work animals and milk and beef producers. Indonesian Agricultural Research and Development Journal 1(1/2):9-10, 24.
Sutedja, P., M. Kota, 1. B. Mantra, and D. Darmadja. 1976. Progress report beberapa performance pada sapi Bali. In Proceedings, Seminar Reproduksi den Performance Sapi Bali, April 5-6, 1976. Dinas Peternakan Daerah Ternak Institut, Bali, Indonesia.
Thahar, A., J. B. Moran, and J. T. Wood. In press. Studies on the haemotology of Indonesian large ruminants. Tropical Animal Health and Production.
Thahar, A., J. B. Moran, and R. E. Soerifpo. In press. Observations on the hair and skin of Indonesian large ruminants and their relationships to heat tolerance. SABRAO Journal.
Vercoe, J. E., and J. E. Frisch. 1977. The importance of voluntary feed intake and metabolic rate to production in different genotypes of cattle in different environments. Pp. I(c)-42 in Proceedings of the 3rd International Congress of the Society for the Advancement of Breeding Researchers in Asia and Oceania, Canberra, Australia.
Yeates, N. T. M., and P. J. Schmidt. 1974. Beef Cattle Production. Butterworths Pty Ltd., Chatswood, Sydney, Australia. 323 pp.
Banteng Cattle Hybrids
Payne, W. J. A., and D. H. L. Rollinson. 1976. Madura cattle. Zeitschrift fur Tiezuchtung und Zuchtungsbiologie 93:89-100.
Mithan
Simoons, F. J., and E. S. Simoons. 1968. A Ceremonial Ox of India: The Mithan in Nature, Culture, and History. The University of Wisconsin Press, Madison, Wisconsin, USA.
Mason, 1. L. In Press. Evolution of Domesticated Animals. Longman, London, England.
Scheurmann, E. 1975. Observations on the behaviour of the mithan (Bibos frontalis Lambert 1837) in captivity. Applied Animal Ethology I :321 -355.
Yak and Yakow
Accolas, J. P., and J. P. Deffontaines. 1970. Quelques donnees sur l'elevage du Yak en Republique Populaire de Mongolie. Pp. 133-141 in The Yak: Its Role in the Economic and Cultural Life of Breeders in Central Asia. Societe d'Ethnozootechnie, 25 Boulevard Arago, 75013 Paris, France.
Bonadonna, T. 1970. Nuove possibilita per la Zootechnia dei tropic). Zootecnica e Veterinaria 25:202-221.
Bonnemaire, J. 1976. Le Yak domestique et son hybridation. Pp. 46-77 in The Yak: Its Role in the Economic and Cultural Life of Breeders in Central Asia. Societe d'Ethnozootechnie, Paris, France.
Dor, R. 1976. Note sur le Yak au Pamir. Pp. 126-131 in The Yak: Its Role in the Economic and Cultural Life of Breeders in Central Asia. Societe d'Ethnozootechnie, Paris, France.
Ekvall, R. B. 1968. Fields on the Hoof: Nexus of Tibetan Nomadic Pastoralism. Holt, Rinehart and Winston, New York, USA.
Epstein, H. 1974. Yak and chauri. World Animal Review 9:8-12.
Epstein, H. 1977. Yak and Yak-cattle hybrids. Chapter 3 in Domestic Animals of Nepal. Holmes and Meier Publishers, Inc., New York, USA, and London, England.
Ivanova, V. V. 1938. K voprosu izucenija plodovitosti samvoc gibridov jaka s rogatym skotom (On the fertility of male yak x cattle hybrids). Izvestiya Akademii Nauk SSSR, Seriya Biologicheskaya 883-884.
Phillips, R. W., R. G. Johnson, and R. T. Moyer. 1945. The livestock of China. Pp. 67-76 in United States Department of State Publication No. 2249; Far Eastern Series 9.
Phillips, R. W., I. A. Tolstoy, and R. G. Johnson. 1946. Yaks and Yak-cattle hybrids in Asia. The Journal of Heredity 37(6): 162-170; 37(7):206-215.
Schley, P. 1967. Der Yak und seine Kreuzung mit dem Rind in der Sowjetunion. Giessener Abhandlungen zur Agrar- und Wirtschaftsforschung des Europaischen Ostens, Vol. 44. Otto Harassowitz, Weisbaden, Federal Republic of Germany. 131 pp.
Shahrani, M. Nazif Mohib. 1979. The Kirghiz and Wakki of Afghanistan. University of Washington Press, Seattle, Washington, USA. 264 pp.
Vlasov, P., S. Gersenzon, and A. Poljakov. 1932. Gibridizacija mezdu jakom i krupnym rogatym skotom (Hybrids between yak and cattle). Problemy Zhivotnovodstva (1):48-57.
White, W. T., R. W. Phillips, and E. C. Elting. 1946. Yaks and Yak-cattle hybrids in Alaska. Journal of Heredity 37:355-358.
Zawadowsky, M. M. 1931. Zebu-Yak hybrids. Journal of Heredity 22:297.
Zuitin, A. I. 1938. New data on the chromosome number in yak (Poephagus grunniens L.). Doklady Akademii Nauk Uzbekskoi SSR 19:201-202.
Wild Banteng
A unique, interesting film showing wild banteng, gaur and kouprey in Cambodia in 1951
is available for hire from The Coolidge Foundation, 38 Standley Street, Beverly, Massachusetts 01915, USA.
Boonsong Lekagul and J. A. McNeely. 1977. Mammals of Thailand. Association for the Conservation of Wildlife, Bangkok, Thailand. 758 pp.
Fischer, H., and E. Scheurmann. 1977. Cytogenetic investigations on domestic and wild animal populations in South and Southeast Asia. Animal Research and Development 6:63-71.
Halder, U. 1975. Okologie und Verhalten des banteng (Bos javanicus) in Java. Eine Feldstudie. Verlag Paul Parey, Hamburg and Berlin, Federal Republic of Germany. 124 pp.
Hoogerwerf, A. 1974. Report on a Visit to Wildlife Reserves in East Java. Mededelingen No. 21. Nederlandsche Commissie voor Internationale Natuurbescherming.
McNeely, J. A. 1975. Wildlife and national parks in the Lower Mekong Basin. Unpub
lished report to the Committee for the Coordination of Investigations of the Lower Mekong Basin, Economic Commission for Asia and the Far East, Bangkok, Thailand.
Sumardja, E. A. 1976. The relationships between banteng Bos javanicus (d'Alton) and vegetation at the Pananjung-Pangandaran Nature Reserve. Research Grant No. 1237/ TF/VI/74. Preliminary Study Internal Report Biotrop. Bogor, Indonesia.
Wind, J., and H. Amir. 1977. Distribution of banteng (Bos javanicus) in Java and Bali, Indonesia. Unpublished ms.
Wind, J., and H. Amir. 1977. Management Plan Baluran Reserve FAO INS/73/013. Nature Conservation and Wildlife Management Project.
Gaur
Boonsong Lekagul. 1965. The gaur. (Bos gaurus). Conservation News of South East Asia 5:7-10.
Boonsong Lekagul and J. A. McNeely. 1977. Mammals of Thailand. Association for the Conservation of Wildlife, Bangkok, Thailand. 758 pp.
Conry, P. J. 1980. The impact of development and the behavioural response of the Malaysian seladang. Pp. 279-286 in Tropical Ecology and Development, Proceedings Fifth International Symposium of Tropical Ecology. International Society of Tropical Ecology, Kuala Lumpur, Malaysia.
Conry, P. J. 1981. Habitat selection and use, movements, and home range of Malayan gaur (Bos gaurus hubbacki) in Central Pahang, Malaysia. M.S. thesis. University of Montana, Missoula, Montana, USA. 120 pp.
Conry, P. J. (In press) Immobilizing Malayan gaur with etorphine. Journal of Wildlife Management.
Fischer, H. 1975. The water buffalo and related species as important genetic resources: their conservation, evaluation and utilization. In The Asiatic Water Buffalo. Proceedings of an International Symposium, Khon Kaen, Thailand. Food and Fertilizer Technology Center, Taipei, Taiwan.
Fischer, H. 1980. Hybrids between Gaur and Gayal and between Gayal and Banteng as zoological rarities in Thailand. Animal Research and Development 12:116-119.
Fradrich, H., and H. G. Klos. 1976. Zur Haltung und Zucht des Gaurs, Bos gaurus. Zoologische Garten 46(6):417-425.
Hussain, M. Khader. 1969. The southern Asiatic gaur or Indian bison (Bos gaurus gaurus H. Smith). Cheetal 12(1):29-47.
Krishnan, M. 1972. An ecological survey of the larger mammals of peninsular India. Journal of the Bombay Natural History Society 69:322-349.
Medway, Lord. 1978. The Wild Mammals of Malaya and Singapore. 2nd Edition. Oxford University Press, Kuala Lumpur, Malaysia. 128 pp.
Momin Khan, M. K. bin 1973. Studies on the seladang (Bos gaurus) in the State of Perak. Malaysian Nature Journal 26:163-169.
Sahai, R. C. 1972. Distribution, composition and herd size of Gaur (Bos gaurus) in Palamau, Bihar. Cheetal 15:60-66.
Schaller, G. B. 1967. The Deer and the Tiger. Chicago University Press, Chicago, USA. 370 pp.
Weigun, L. E. 1971. The last refuge. Malaysian Nature Journal 24:132-137.
Weigun, L. E. 1972. The problems in the preservation of the seladang in the Malaysian National Park. M.S. thesis. Michigan State University, East Lansing, Michigan, USA. 55 pp
Kouprey
Boonsong Lekagul and J. A. McNeely. 1977. Mammals of Thailand. Association for the Conservation of Wildlife, Bangkok, Thailand. 758 pp.
Coolidge, H. J. 1940. The Indo-Chinese Forest Ox or Kouprey. Memoirs, Museum of Comparative Zoology at Harvard College 54(6):421-431.
Coolidge, H. J. 1955. The Forest Ox or Kouprey of South East Asia. P. 109 in Breeding Beef Cattle for Unfavorable Environments, A. O. Rhoad, ed. University of Texas Press, Austin, Texas, USA.
Neese, H.C. 1976. Kouprey along the Laos/Cambodia border area. Unpublished report to the New York Zoological Society, 185 and Southern Blvd., New York, New York 10460, USA.
Pfeffer, P., and O. Kim-San. 1967. Le kouprey, Bos(Bibos) sauvelli, Urbain, 1937: discussion systematique et status actuel. Hypothese sur l'origine du zebu (Bos indicus). Mammalia 31(4):521-536.
Pfeffer, P. 1969. Considerations sur l'ecologie des forets claires du Cambodge oriental. Terre et Vie 23(1):3-24.
Wharton, C. H. 1957. An Ecological Study of the Kouprey. Novibos sauveli (Urbain). Monograph No. 5. Institute of Science and Technology, Manila, Philippines. 111 pp.
Anoas
Dolan, J. M. 1965. Breeding of the lowland anoa in the San Diego Zoological Garden. Zeitschrift Saugetierekunde 30(4):241-248.
Duplaix-Hall, N., ed. 1975. International Zoo Yearbook 15. Zoological Society of London
Fradrich, H. 1973. Einige Bemerkungen uber den Anoa. Zeitschrift des Kolner Zoo 16(3):101-105.
Fischer, H., and H. Hohn. 1976. Der Karyotyp eines weiblichen Tamarau (Anea mindorensis) [The Karyotype of a Female Tamaraw (Anoa mindorensis)]. Giessener Beitrage zur Erbpathologie und Zuchthygiene 6:173.
Groves, C. P. 1969. Systematics of the anoa (Mammalia, Bovidae) Beaufortia 17(223): 1-12.
Koulischer, L., J. Tyskens, and J. Mortelsmanns. 1972. The chromosomes of a male specimen of Anoa depressicornis quarlesi. Acta Pathologica, Antwerp 56:21.
Scheurmann, E., H. Hohn, and H. Fischer. 1977. The karyotype of a male Bubalus (Anoa) depressicornis quarlesi Ouwens 1911. In Symposium, Domesticated Animal Cytogenetics, Jouy en Josas.
Tamaraw
Alvarez, J. B., Jr. 1970. Philippines tamaraw, here to stay. IUCN Publication, New Series 18:46-51.
Grimwood, l. R. 1975. National Parks and Wildlife Conservation in the Philippines. FAO: PHI/72/006. UNDP/FAO, Rome, Italy.
Harrisson, T. 1969. The tamaraw and its survival. Conservation News of South East Asia 8:60-61, 70.
Kuchn, D. 1975. Tamaraw at Mt. Iglit Came Refuge, Philippines. Tigerpaper 2(3):26.
Sitwell, N. 1975. On the track of the tamaraw. Wildlife 17:428-430.
Talbot, L. M., and M. H. Talbot. 1966. The tamaraw, Bubalus mindorensis (Heude). Observations and recommendations. Mammalia 30(1): 1 - 12.
Bearded
Pig
Allen, E. F. 1948. The bearded pig. Malaysian Nature Journal 3(2):98-99.
Cranbrook, Earl of. 1979. A review of domestic pig remains from archaeological sites in
Diong, C. H. 1973. Studies of the Malayan wild pig in Perak and Johore. Malaysian Nature Journal26(2):120-151.
Croves, C. P. 1981. Ancestors for the Pigs, Taxonomy and Phylogeny of the Cenus Sus. Department of Prehistory Technical Bulletin No. 3. Australian National University Press, Canberra, Australia. 96 pp.
Hertwig, P. 1936. Artbastarde bei Tieren. Handbuch Vererb Wissenschaften 2B:21.
Hislop, J. A. 1949. Field notes on the bearded pig. Malaysian Nature Journal 4(2):62-64.
Hislop, J. A. 1952. More about the bearded pig. Malaysian Nature Journal 7:22-23.
International Union for the Conservation of Nature. 1972. Red Data Book 1: Mammalia. IUCN, Cland, Switzerland. (This is a loose-leaf binder that is updated from time to time.)
Kempe, J. E. 1948. The riddle of the bearded pig. Malaysian Nature Journal 3(1):36-42.
Lotsy, J. P. 1922. Die Aufarbeitung des kuhn'schen kreuzungsmaterials im Institut fur Tierzucht der Universitat Halle. Cenetica 4:32-61.
MacKinnon, J. 1981. The distribution and status of wild pigs in Indonesia. IUCN/SSC Pigs and Peccaries Specialist Group. Unpublished report. 9 pp.
Medway, Lord. 1978. The Wild Mammals of Malaya and Singapore, 2nd Edition. Oxford University Press, Kuala Lumpur, Malaysia.
Mohr, E. 1960. Wilde Schweine. Die Neue Brehm-Biicherei, A. Ziemsen Verlag, Wittenberg Lutherstadt, Federal Republic of Cermany.
Oliver, W. L. R. 1981. First written report. IUCN/SSC Pigs and Peccaries Specialist group. Unpublished report. 14 pp.
Pfeffer, P. 1959. Bologie et migrations du sanglier de Borneo (Sus barbatus Muller 1869). Mammalia 23:277-303.
Sulawesi Warty Pig
Appelman, F. J. 1955. Uber Sus celebensis Muller und Schlegel. Der Zoologische Garten 21:152-156.
Catibog-Sinha, C. S. 1981. The quantity and quality of wild food plants and the depredation of wild pigs in the Philippines. Ph.D. thesis. Oklahoma State University, Stillwater Oklahoma, USA.
Croves, C. P. 1981. Ancestors for the Pigs, Taxonomy and Phylogeny of the Cenus Sus. Department of Prehistory Technical Bulletin No. 3. Australian National University Press, Canberra, Australia. 96 pp.
Hooijer, D. A. 1968. Pleistocene Vertebrates from Celebes. 8. Sus celebensis Muller and Schlegel, 1845. Beaufortia 16:215-218.
MacKinnon, J. 1981. The distribution and status of wild pigs in Indonesia. IUCN/SSC Pigs and Peccaries Specialist Croup. Unpublished report. 9 pp.
Mohr, E. 1960. Wilde Schweine. Die Neue Brehm-Bucherei, A. Ziemsen Verlag, Wittenberg Lutherstadt, Federal Republic of Germany.
Oliver, W. L. R. 1981. Interim report. IUCN/SSC Pigs and Peccaries Specialist Group. Unpublished report. 14, pp.
Javan Warty Pig
Blouch, R. A. 1979. Proposed Bawean Island Wildlife Reserve Management Plan. World Wildlife Fund, Report to Directorate of Nature Conservation, Indonesia. Unpublished report. 63 pp.
Blouch, R. A. 1981. Results of a preliminary search for Sus verrucosus in the Mt. Arjuna area. World Wildlife Fund, Meru Betiri Project 1024. Unpublished report. 4 pp.
Groves, C. P. 1981. Ancestors for the Pigs, Taxonomy and Phylogeny of the Genus Sus. Department of Prehistory Technical Bulletin No. 3. Australian National University Press, Canberra, Australia. 96 pp.
Hoogerwerf, A. 1970. Udjung Kulon—the Land of the Last Javan Rhinoceros. E. J. Brill, I.eiden. The Netherlands. Pp. 330-349.
MacKinnon, J. 1981. The distribution and status of wild pigs in Indonesia. IUCN/SSC Pigs and Peccaries Specialist Group. Unpublished report. 9 pp.
Oliver, W. L. R. 1981. First written report. IUCN/SSC Pigs and Peccaries Specialist Group. Unpublished report. 14 pp.
Pigmy Hog
Bosma, A. A., W. L. R. Oliver, and A. A. MacDonald. In press. The karyotype, including g- and c- banding patterns, of the pigmy hog Sus (Porcula) salvanius (Suidae, Mammalia).
Groves, C. T. 1981. Ancestors for the Pigs, Taxonomy and Phylogeny of the Genus Sus. Department of Prehistory Technical Bulletin No. 3. Australian National University Press, Canberra, Australia. 96 pp.
Oliver, W. L. R. 1980. The Pigmy Hog: The Biology and Conservation of the Pigmy Hog Sus (Porcula) salvanius, and the Hispid Hare, Caprolagus hispidus. Special Scientific Report No. I. Jersey Wildlife Preservation Trust. 93 pp.
Oliver, W. L. R. 1981. Pigmy hog and hispid hare: further observations of the continuing decline (or, a lament for Barnadi and a good cause for skepticism). The Dodo, Journal of the Jersey Wildlife Preservation Trust 18:10-20.
Babirus a
Bosma, A. A., 1980. The karyotype of the babirusa (Babyrousa babyrussa); karyotype evolution in the Suidae. Fourth European Colloquium Cytogenetics Domestic Animals 1980:238-241.
Bosma, A. A., and De Haan, N. A. 1981. The karyotype of Babrousa babyrussa (Suidae, Mammalia) Acta Zoologica et Pathologica Antverpiensia 76: 17-27.
Davis, D. D. 1940. Notes on the anatomy of the babirusa. Field Museum of Natural History-Zoology 22(5):363-411.
Fradich, H. 1974. A comparison of behaviour in the suidae. Pp. 133-144 in The Behaviour of Ungulates and Its Relation to Management. V. Ceist and F. Walther, eds. IUCN Publication No. 24.
Groves, C. P. 1981. Ancestors for the Pigs, Taxonomy and Phylogeny of the Genus Sus. Department of Prehistory Technical Bulletin, No. 3. Australian National University Press, Canberra, Australia. 96 pp.
Croves, C. P. 1980. Notes on the systematics of Babyrousa (Artiodactyla, Suidae). Zoologische Mededelingen, Deel 55(3):29-46.
MacKinnon, J. 1981. The structure and function of the tusks of babirusa. Mammal Review 1 1(1):37-40.
MacKinnon, J. 1981. The distribution and status of wild pigs in Indonesia. IUCN/SSC Pigs and Peccaries Specialist Group. Unpublished report. 9 pp.
Oliver, W. L. R. 1981. First written report. IUCN/SSC Pigs and Peccaries Specialist Group. Unpublished report. 14 pp.
1
Selmier, V. J. 1978. Only in Indonesia: the babirusa. Unpublished report to Directorate of Nature Conservation (P.P.A.), Bogor, Indonesia. 40 pp.
Because Asia contains many livestock breeds about which little is known, an expert committee on Animal Genetic Resources has been recently formed by the Society for the Advancement of Breeding Researchers in Asia and Oceania (SABRAO). Its chairman is Professor J. S. F. Barker of the University of New England, Armidale, New South Wales 2351, Australia.
The Species Survival Commission of the International Union for the Conservation of Nature has a Wild Cattle Specialist Group that concerns itself with wild banteng, gaur, and kouprey. Its chairman is Mr. Mohd. Khan bin Momin Khan, Director-General, Wildlife and National Parks, West Malaysia, Kompleks Pejabat-Pejabat Kerajaan, Block K-20, Jalan Duta, Kuala Lumpur, Malaysia.
The following have particular knowledge of the animals described in this book, have contributed to the various chapters, and (in most cases) have agreed to provide advice to bona fide researchers wishing to study further the animals in this report.
Domesticated Banteng
Ida Bagus Arka, Faculty for Veterinary Science and Animal Husbandry, Udayana University, Jalan Sudirman Sanglah, Denpasar, Bali, Indonesia J. S. F. Barker, Department of Animal Science, University of New
England, Armidale, New South Wales 2351, Australia
W. Bongers, Department of Nature Conservation, Wageningen Agricultural University, Ritzema Bosweg 32a, 6703AZ Wageningen, The Netherlands David Butcher, Western Plains Zoo, Box 813, Dubbo, New South Wales
2830, Australia (Banteng are available for research projects and zoos.
Quarantine for shipments between Australia and most countries are
straightforward)
J. K. Camoens, Livestock Specialist, Asian Development Bank, P.O. Box 789, Manila, Philippines 2800
David Deppner, 1603 Dayton Road, Hyattsville, Maryland 20783, USA
C. Devendra, Malaysian Agricultural Research and Development Institute (MARDI), Serdang, Selangor, Malaysia
J.-M. Duplan, Institut Technique de l'Elevage Bovin, Section Amelioration Genetique, Maison Nationale des Eleveurs, 149, rue de Bercy F, 75595 Paris Cedex 12, France
H. Fischer, Institute of Tropical Veterinary Medicine, 63 Giessen, Wilhelmstrasse 15, Federal Republic of Germany
S. I. Furtado, Universiti Malaya, Lembah Pantai, Kuala Lumpur 22-11, Malaysia.
John Hodges, FAO, Via delle Terme di Caracalla, 00100 Rome, Italy
H. Huitema, Overzicht 60, 6862 CT, Oosterbeek, The Netherlands
Graham W. M. Kirby, Principal Animal Production Officer, Department of Primary Production, P.O. Box 4160, Darwin, Northern Territory 5790, Australia
Moh. Zain Katoe, P.T. United Livestock, Jalan Bau Massepe 472, ParePare, South Sulawesi, Indonesia
H. -G. Klos, Director, Zoologischer Garten Berlin, Hardenbergplatz 8 , 1000 Berlin 30, Federal Republic of Germany
Prof. Kusmat, Dean, Faculty of Veterinary Medicine, Institut Pertanian Bogor, Jalan Oto Iskandardinata, Bogor, Indonesia
Ian L. Mason, 8 Ramsay Garden, Edinburgh, EH1 1NA, Scotland
Robert E. McDowell, Department of Animal Science, Frank B. Morrison Hall, Cornell University, Ithaca, New York 14853, USA
G. Montsma, Department of Animal Production, Wageningen Agricultural University, Ritzema Bosweg 32a, 6703 AZ Wageningen, The Netherlands
J. B. Moran, Senior Research Officer, Animal and Irrigated Pastures Research Institute, Kyabram, Victoria 3620, Australia
Jan Nari, Director of Central Research Institute for Animal Science, Jalan Raya Pajajaran, Bogor, Indonesia
I. M. Nitis, Department of Animal Nutrition and Tropical Pasture Production, Udayana University, Jalan Sudirman Sanglah, Denpasar, Bali, Indonesia
W. J. A. Payne, 91 Bedwardine Road, London SE19 3AY, England
Donald L. Plucknett, Consultative Group on International Agricultural Research, 1818 H Street, Washington, D.C. 20433, USA
Research Institute for Animal Production, P.O. Box 123, Bogor, Indonesia (Amber Roesyat, Ian Fletcher, Dennis Hoffmann, P. Sitorus, Subandriyo, M. Zulbardi)
David W. Robinson, International Programs, University of California, Davis, California 95616, USA
D. H. L. Rollinson, Animal Production and Health Division, FAO, Via delle Terme di Caracalla, 00100 Rome, Italy
G. Seifert, CSIRO, Tropical Cattle Research Centre, P.O. Box 5545, Rockhampton Mail Centre, N. Rockhampton, Australia
C. David Simpson, Senior Extension Specialist, Veld, Pastures and Wildlife, Agritex Box 363, Bulawayo, Zimbabwe
A. J. Smith, Tropical Animal Health, Royal (Dick) School of Veterinary Studies, University of Edinburgh, Summerhall, Edinburgh, EH9 1QH Scotland
Dukut Sularsasa, Department of Tropical Veterinary Science, James Cook University, Townsville, Queensland 4811, Australia
M. Syamsul Arifin, Jalan Amin Jakfar 41, Pamekasan Madura, Jatim, Indonesia
Taronga Zoo, P.O. Box 20, Mosman, New South Wales 2088, Australia (T. Finnie and J. L. Throp)
Frank M. Thompson, Wild Animal Brokers, 15605 S. R. #64, Bradenton, Florida 33508, USA
Allen D. Tillman, 523 West Harned Place, Stillwater, Oklahoma 74074, USA
Don Tulloch, P.O. Box 38841, Winnellie, Northern Territory 5789, Australia
Helen Newton Turner, P.O. Box 184, North Ryde, New South Wales 2113, Australia
Antoon de Vos, P.O. Box 34, Whitford, Auckland, New Zealand
Robert Warren, Department or Range and Wildlife Management, Texas Tech University, Lubbock, Texas 79409, USA
Everett J. Warwick, The Rockefeller Foundation, P.O. Box 63, Yogyakarta, D.I.Y., Indonesia
John Woodward, 35AI, Sultan Hasanudin, Ujung Pandang, South Sulawesi, Indonesia
Zoos with Domesticated Banteng
Antwerp, Belgium (Royal Zoological Society of Antwerp)
Askaniya-Nova, USSR (Zoologicheskii Park Askaniya-Nova)
Bangkok, Thailand (Dusit Zoological Park; banteng-mithan hybrid)
Berlin, Federal Republic of Germany (Zoologischer Garten)
Berlin, German Democratic Republic (Tierpark Berlin)
Copenhagen, Denmark (Zoologisk Have)
Dubbo, New South Wales, Australia (Western Plains Zoo)
Jakarta, Indonesia (Kebun Binatang Ragunan)
Kiev, USSR (Kievskii Zoologicheskii Park)
Madrid, Spain (Zoo de la Casa de Campo; mithan-banteng hybrid)
Pretoria, South Africa (National Zoological Gardens of South Africa)
Rome, Italy (Giardino Zoologico e Museo de Zoologia del Comune de Rome and Zoorama)
Rotterdam, The Netherlands (Stichting Koninklijke Rotterdamse Diergaarde; 2 male, 2 female, may be wild form)
Surabaya, Indonesia (Kebun Binatang Surabaya)
Yogyakarta, Indonesia
Banteng-Cattle Hybrids
Abubakar Aldjufri, Secretary, Asian Livestock Business Club, Kebun Pala I/79B Tanah Abang, Jakarta, Indonesia
Saleh Aldjufri, Jalan Jakarta 42, Surabaya, Jawa Timur, Indonesia
David Deppner, 1603 Dayton Rd., Hyattsville, Maryland 20783, USA
H. Huitema, Overzicht 60, 6862 CT, Oosterbeek, The Netherlands
Graham W. M. Kirby, Principal Animal Production Officer, Department of Primary Production, P.O. Box 4160, Darwin, Northern Territory 5790, Australia
J. B. Moran, Senior Research Officer, Animal and Irrigated Pastures Research Institute, Kyabram, Victoria 3620, Australia
W. J. A. Payne, 91 Bedwardine Road, London SE19 3AY, England
David W. Robinson, International Programs, University of California, Davis, California 95616, USA
D. H. L. Rollinson, Animal Production and Health Division, FAO, Via delle Terme di Caracalla, 00100 Rome, Italy
B. Wheeler, 1000 Shady Lane, College Station, Texas 77840, USA
Mithan
Chaichana Satrulee, Dusit Zoological Park, Bangkok, Thailand
H. Fischer, Institute of Tropical Veterinary Medicine, 63 Giessen, Wilhelmstrasse 15, Federal Republic of Germany
C. von Furer Haimendorf, School of Oriental and African Studies, University of London, London WC1, England
Charles G. Hickman, Livestock Consultant, A. Mithat Efendi Sokak No. 36/11, Cankaya, Ankara, Turkey
Frederick J. Simoons, 140 Bartlett Ave., Woodland, California 95695, USA
Zoos with Mithan (Gayal)
Aalborg, Denmark (Aalborg Zoologiske Have)
Amsterdam, The Netherlands (Stichting Koninklijk Zoologisch)
Arnhem, The Netherlands (Burgers' Zoo and Safari)
Bangkok, Thailand (Dusit Zoological Park)
Berlin, German Democratic Republic (Tierpark Berlin)
Calcutta, India
Cologne, Federal Republic of Germany (Aktiengesellschaft Zoologischer Garten)
Heidelberg, Federal Republic of Germany (Tiergarten Heidelberg)
Kolmarden, Sweden (Koemardens Djurpark)
Madrid, Spain (Zoo de la Casa de Campo; mithan-banteng hybrid)
Rabat, Morocco (Pare Zoologique National)
Vienna, Austria (Schonbrunn Zoo)
Tallin, USSR (Tallinna Loomaaed)
Yak
Joseph Bonnemaire, Ecole Nationale Superieure des Sciences Agronomiques Appliquees, 26, Bd. Docteur Petitjean, 21100 Dijon, France
Corneille Jest, Centre National de la Recherche Scientifique, 1, Place Artistide-Briand, 92190 Meudon, France
R. A. Kazarova, Director, Riga Zoological Garden, Meza prospekt 1, Riga 14 226014, Latvian SSR, USSR
K. K. Panday, Swiss Association for Technical Assistance (SATA), Box 113, Kathmandu, Nepal
Ralph W. Phillips, The Representative, Apartment 810, 1101 South Arlington Ridge Road, Arlington, Virginia 22202, USA
Patrick J. Robinson, Department of Agricultural and Forest Sciences, University of Oxford, Commonwealth Forestry Institute, South Parks Road, Oxford OX1 3RB, England
George B. Schaller, New York Zoological Society, 185 and Southern Blvd., New York, New York 10460, USA
T. B. Singh, Director General, Department of Livestock, HMG, Harihar Bhawan, Pulchowk, Laitpur, Nepal
Societe d'Ethnozootechnie, 25, Boulevard Arago, 75013 Paris, France (R. Laurans)
Zoos with Yak
Antwerp, Belgium (Royal Zoological Society of Antwerp)
Arnhem, The Netherlands (Burgers' Zoo and Safari)
Baltimore, Maryland, USA
Bronx, New York, USA (New York Zoological Park)
Chicago, Illinois, USA (Brookfield Zoo)
Denver, Colorado, USA (Denver Zoological Gardens)
Detroit, Michigan, USA (Detroit Zoological Park)
Evansville, Indiana, USA (Meeker Park Zoo)
Granby, Quebec, Canada (Societe Zoologique de Granby, Inc.)
Grand Rapids, Michigan, USA (John Ball Zoological Gardens)
Kansas City, Missouri, USA (Kansas City Zoological Gardens)
Knoxville, Tennessee, USA (Knoxville Municipal Zoo)
London, England
Rochester, New York, USA (Seneca Park Zoo)
Rotterdam, The Netherlands (Stichting Koninklijke Rotterdamse Diergaarde)
San Diego, California, USA (San Diego Wild Animal Park)
Sioux Falls, South Dakota, USA (Great Plains Zoo)
South Bend, Indiana, USA (Potawatomi Park Zoo)
Syracuse, New York, USA (Burnet Zoo)
Toronto, Ontario, Canada (Metro Toronto Zoo)
Utica, New York, USA (Utica Zoo)
Wichita, Kansas, USA (Sedgwick County Zoo)
West Orange, New Jersey, USA (Turtleback Zoo)
Winnipeg, Manitoba, Canada (Assiniboine Park Zoo)
Yakows
Joseph Bonnemaire, Ecole Nationale Superieure des Sciences Agronomiques Appliquees, 26, Bd. Docteur Petitjean, 21100 Dijon, France
Department of Agriculture, Hari Har Bhawan, Pulchok, Lalitpur, Kathmandu, Nepal
Department of Animal Husbandry and Veterinary Science, Kathmandu, Nepal (Tewari and Burathoky)
Director of Livestock Development, Peking, China
Professor Karl Fredge, Department of Genetics, University of Uppsala, Uppsala, Sweden
Indian Veterinary Research Institute, P.O. Izatnagar 243 122, Uttar Pradesh, India
Livestock Division, National Agricultural Research Center, Islamabad, Pakistan
National Sheep and Yak Development Project, Dechhen Pelrithang, P.O. Bumthang, Bhutan
Ralph W. Phillips, The Representative, Apartment 810, 1101 South Arlington Ridge Road, Arlington, Virginia 22202, USA
Patrick J. Robinson, Department of Agricultural and Forest Sciences, University of Oxford, Commonwealth Forestry Institute, South Parks Road, Oxford OX1 3RB, England
Whipsnade Zoo, Whipsnade, England
Bruce A. Young, Department of Animal Science, University of Alberta, Edmonton, Alberta TOG 2P5, Canada
Wild Banteng
H. Amir, c/o Ministry of Development Supervision and the Environment, JalanMirdekaBarat 15, Jakarta, Indonesia
J. H. Blower, National Parks Project, c/o UNDP, Box 650, Rangoon, Burma
Bonsong Lekagul, Association for the Conservation of Wildlife, No. 4 Customhouse Road, Bangkok, Thailand
Chaichana Satrulee, Dusit Zoological Park, Bangkok, Thailand
Z. Coto, Tropical Forest Biology, SEAMEO-BIOTROP, P.O. Box 17, Bogor, Indonesia
Forest Department, P.O. Box 311, Sandakan, Sabah, Malaysia (Patrick Andau, Assistant Chief Game Warden)
I. R. Grimwood, P.O. Box 45079, Nairobi, Kenya
C. P. Groves, Department of Prehistory and Anthropology, The Australian National University, P.O. Box 4, Canberra, ACT 2600, Australia
U. Halder, Swiss League for Nature Conservation, P.O. Box 73, CH4020 Basel, Switzerland
H. Huitema, Overzicht 60, 6862 CT, Oosterbeek, The Netherlands
F. Wayne King, Director and Professor, Florida State Museum, University of Florida, Gainesville, Florida 32601, USA
Lembaga Biologi Nasional (LBN-LIPI), Bogor, Indonesia
John A. Lukas, White Oak Plantation, Rt. 3 Box 224, Yulee, Florida 32097, USA
Jeffrey A. McNeely, Executive Officer, Commission on National Parks and Protected Areas, International Union for Conservation of Nature and Natural Resources, Avenue du Mont-Blanc, 1196 Gland, Switzerland
John Payne, Wildlife Section, Forest Department, P.O. Box 311, Sandakan, Sabah, Malaysia
Somtob Norapuckprutikorn, Captive Propagation Section, Wildlife Conservation Division, Royal Forest Department, Bangkhen, Bangkok 9, Thailand
B. Wheeler, 1000 Shady Lane, College Station, Texas 77840, USA
M. H. Woodford, c/o FORW, FAO, Via delle Terme di Caracalla, 00100 Rome, Italy
Zoos with Wild Banteng
Albuquerque, USA (Rio Grande Zoological Park)
Amsterdam, The Netherlands (Stichting Koninklijk Zoologisch)
Bangkok, Thailand (Dusit Zoological Park)
Berlin, Federal Republic of Germany (Zoologischer Garten)
Chicago, Illinois, USA (Brookfield Zoo)
Cologne, Federal Republic of Germany (Aktiengesellschaft Zoologischer Garten)
Copenhagen, Denmark (Zoologisk Have)
Darmstadt, Federal Republic of Germany (Vivarium Darmstadt)
Dortmund, Federal Republic of Germany (Tierpark Dortmund)
Dresden, German Democratic Republic (Zoologischer Garten Dresden)
Frankfurt, Federal Republic of Germany
Jakarta, Indonesia (Kebun Binatang Ragunan)
San Diego, California, USA
St. Louis, Missouri, USA (St. Louis Zoological Park)
Surabaya, Indonesia (Kebun Binatang Surabaya)
Yogyakarta, Indonesia
Yulee, Florida, USA (White Oak Plantation)
Gaur
John Aspinal, Howlett's Wildlife Park, near Canterbury, Kent, England
Boonsong Lekagul, Association for the Conservation of Wildlife, No. 4 Customhouse Road, Bangkok, Thailand
Paul Conry, Division of Aquatic and Wildlife Resources, Department of Agriculture, P.O. Box 23367, GMF Guam 96921, USA
H. Fischer, Institute of Tropical Veterinary Medicine, 63 Giessen, Wilhelmstrasse 15, Federal Republic of Germany
I. R. Grimwood, P.O. Box45079, Nairobi, Kenya
Mohd Khan bin Momin Khan, Director-General, Department of Wildlife and National Parks, P.O. Box 611, Kuala Lumpur, Malaysia
R. A. Kazarova, Director, Riga Zoological Garden, Meza prospekt 1, Riga 14 226014, Latvian SSR, USSR
H.-G. Klos, Director, Zoologischer Garten Berlin, Hardenbergplatz 8, 1000 Berlin 30, Federal Republic of Germany
Adrian G. Marshall, Department of Zoology, University of Aberdeen, Aberdeen, Scotland
George B. Schaller, New York Zoological Society, 185 and Southern Blvd., New York, New York 10460, USA
Lee Simmons, Omaha Zoo, Omaha, Nebraska 68108, USA
Janet Stover, Bronx Zoo, 185 and Southern Blvd., New York, New York 10460, USA
Frank M. Thompson, Wild Animal Brokers, 15605 S. R. #64, Bradenton, Florida 33508, USA
M. H. Woodford, c/o FORW, FAO, Via delle Terme di Caracalla, 00100 Rome, Italy
Zoos with Guar
The Berlin Zoo was assigned by the IUCN in cooperation with the
lUDZG to keep the guar studbook records.
Bangkok, Thailand (Dusit Zoological Park)
Berlin, Federal Republic of Germany (Zoologischer Garten)
Bronx, New York, USA (New York Zoological Park; 7 male, 3 female)
Brownsville, Texas, USA (Gladys Porter Zoo; 5 male, 10 female)
Copenhagen, Denmark (Zoologisk Have; 1 male, 2 female)
Holiday Island, Eureka Springs, Arkansas, USA
Hyderabad, Andhra Pradesh, India (Nehru Zoological Park)
Ivey, Wilburton, Oklahoma, USA
Kansas City, Missouri, USA (Kansas City Zoological Gardens; I male)
Kings Mill, Ohio, USA (Wild Animal Safari,; 1 male)
Kuala Lumpur, Malaysia
London, England
Los Angeles, California, USA (The Los Angeles Zoo; I male, 3 female)
Memphis, Tennessee, USA (Memphis Zoo and Aquarium; I male, 2 female)
Munich, Federal Republic of Germany (Munchener Tierpark Hellabrunn Ag)
Mysore, Karnataka, India
Oklahoma City, Oklahoma, USA (Oklahoma City Zoo; 7 male, 7 female)
Omaha, Nebraska, USA (Henry Doorly Zoological Gardens; 7 male, 15 female)
Riga, Latvian SSR, USSR (Riga Zoological Garden)
San Diego, California, USA (San Diego Wild Animal Park; 6 male, 6 female)
South Bend, Indiana, USA (Potawatomi Park Zoo; 1 male)
St. Louis, Missouri, USA (St. Louis Zoological Park; 1 male, 2 female)
Toronto, Ontario, Canada (Metro Toronto Zoo; 3 male, 1 female)
Vienna, Austria (Schonbrunn Zoo)
Kouprey
Boonsong Lekagul, Association for the Conservation of Wildlife, No. 4 Customhouse Road, Bangkok, Thailand
Harold Coolidge, 38 Standley Street, Beverly, Massachusetts 01915, USA
Jeffrey A. McNeely, Executive Officer, Commission on National Parks and Protected Areas, International Union for Conservation of Nature and Natural Resources, Avenue du Mont-Blanc, 1196 Gland, Switzerland
Harvey C. Neese, P.O. Box 332, Troy, Idaho 83871, USA
David Anthony Parkinson, White House, San Roque 1, San Jose, Occidental Mindoro, Philippines
Pierre Pfeffer, Zoologie, Mammiferes et Oiseaux Museum National d'Histoire Naturelle 55, rue de Buffon 75005 Paris, France
A. Kelly Shelton, 211-A North Cordova, Burbank, California 91505, USA
Henry Stoddard, Branford Veterinary Clinics, Inc., P.O. Box 548, Branford, Florida 32008, USA
Charles H. Wharton, Institute of Ecology, University of Georgia, Athens, Georgia 30602, USA (Rt. 2, Clayton, Georgia 30525)
World Conservation Centre, Avenue du Mont-Blanc, CH 1196 Gland, Switzerland (David Mitchell, Elizabeth Kemf)
M. H. Woodford, c/o FORW, FAO, Via delle Terme di Caracalla, 00100 Rome, Italy
Zoos with Kouprey
None
Anoas
J. M. Dolan, San Diego Zoological Garden, San Diego, California 92112, USA
H. Fischer, Institute of Tropical Veterinary Medicine, 63 Giessen, Wilhelmstrasse 15, Federal Republic of Germany
C. P. Groves, Department of Prehistory and Anthropology, The Australian National University, P.O. Box 4, Canberra, ACT 2600, Australia
Zoos with Anoas
Antwerp, Belgium (Royal Zoological Society of Antwerp; mountain anoa)
Berlin, Federal Republic of Germany (Zoologischer Garten; mountain anoa)
London, England (lowland anoa)
Leipzig, German Democratic Republic (Zoologischer Garten Leipzig; mountain anoa)
Jakarta, Indonesia (Kebun Binatang Ragunan)
Rotterdam, The Netherlands (Stichting Koninklijke Rotterdamse Diergaarde; 1 male, 3 female)
San Diego, California, USA (San Diego Zoological Garden; 1 male)
San Diego, California, USA (San Diego Wild Animal Park; 1 male)
Surabaya, Indonesia (Kebun Binatang Surabaya)
Sydney, New South Wales, Australia (Taronga Park Zoo)
Tamaraw
Department of Natural Resources, Quezon City, Manila, Philippines (J. B. Alvarez, Jr., Assistant Director)
H. Fischer, Institute of Tropical Veterinary Medicine, 63 Giessen, Wilhelmstrasse 15, Federal Republic of Germany
I. R. Grimwood, P.O. Box 45079, Nairobi, Kenya
Natural Resources Conservation Office, Ministry of Natural Resources, Visayas Avenue, Diliman, Quezon City, Philippines
David Anthony Parkinson, White House, San Roque 1, San Jose, Occidental Mindoro, Philippines
Ian Player, P.O. Box 192, Howick 3290, Natal, South Africa
N. Sitwell, c/o WWF(UK), 11-13 Ockford Rd., Godalming, Surrey GU7 lQU, England
L. M. Talbot, 6656 Chilton Court, McLean, Virginia 22101, USA
Forest Research Institute, College, Laguna 3720, Philippines
Bearded Pig
Julian Oliver Caldecott, Sub-Department of Veterinary Anatomy, University of Cambridge, Downing Street, Cambridge CB2 3DY, England
C. P. Groves, Department of Prehistory and Anthropology, The AustralianNationalUniversity, P.O. Box4, Canberra, ACT 2600, Australia
Alastair A. Macdonald, Department of Anatomy, Royal (Dick) School of Veterinary Studies, University of Edinburgh, Edinburgh EH9 10H, Scotland, United Kingdom
J. MacKinnon, WWF/IUCN Representative, P.O. Box 133, Bogor, Indonesia
National Parks and Wildlife Office, Sarawak Forest Department, Jalan Gartak, Kuching, Sarawak, Malaysia (Kron Aken, David Labang, Mike Kavanagh)
W. L. R. Oliver, Jersey Wildlife Preservation Trust, Les Augres Manor, Jersey, Channel Islands, United Kingdom
John Payne, Wildlife Section, Forest Department, P.O. Box 311, Sandakan, Sabah, Malaysia
M. H. Woodford, c/o FORW, FAO, Via delle Terme di Caracalla, 00100 Rome, Italy
Zoos with Bearded Pig
Singapore, Singapore
Manila, Philippines
Sulawesi Warty Pig
C. Catibog-Sinha, Outdoor Recreation and Wildlife Research Division, Forest Research Institute, College, Laguna 3720, Philippines
C. P. Groves, Department of Prehistory and Anthropology, The AustralianNationalUniversity, P.O. Box4, Canberra, ACT 2600, Australia
Alastair A. Macdonald, Department of Anatomy, Royal (Dick) School of Veterinary Studies, University of Edinburgh, Edinburgh EH9 10H, Scotland, United Kingdom
J. Mackinnon, WWF/TUCN Representative, P.O. Box 133, Bogor, Indonesia
W. L. R. Oliver, Jersey Wildlife Preservation Trust, Les Augres Manor, Jersey, ChanneL Islands, United Kingdom
M. H. Woodford, c/o FORW, FAO, Via delle Terme di Caracalla, 00100 Rome, Italy
Zoos with Sulawesi Warty Pig
None.
Javan Warty Pig
Raleigh A. Blouch, 2125 Forest Glen, Lansing, Michigan 48906, USA
A. A. Bosma, Department of Functional Morphology, Faculty of Veterinary Sciences, State University Utrecht, P.O. Box 80157, 3508 TD Utrecht, The Netherlands
Directorate of Nature Conservation (PPA), Jalan Ir. H. Juanda 9, Bogor, Indonesia
C. P. Groves, Department of Prehistory and Anthropology, The Australian National University, P.O. Box 4, Canberra, ACT 2600, Australia
Lembaga Biologi Nasional (LBN-LIPl), Bogor, Indonesia
Alastair A. Macdonald, Department of Anatomy, Royal (Dick) School of Veterinary Studies, University of Edinburgh, Edinburgh EH9 10EI, Scotland, United Kingdom
J. MacKinnon, WWF/IUCN Representative, P.O. Box 133, Bogor, Indonesia
W. L. R. Oliver, Jersey Wildlife Preservation Trust, Les Augres Manor, Jersey, Channel Islands, United Kingdom
Werner Pauwels, Pfeffingerstrasse 48, CM-053, Basel, Switzerland
Perum Perhutani, Forest State Corporation, Jalan Jendral Gatot Subroto 17-18, Post Box 111, Jakarta, Indonesia
M. H. Woodford, c/o FORW, FAO, Via delle Terme di Caracalla, 00100 Rome, Italy
Zoos with Javan Warty Pig
Jakarta, Indonesia (Kebun Binatang Ragunan)
Surabaya, Indonesia (Kebun Binatang Surabaya)
Pigmy Hog
A. A. Bosma, Department of Functional Morphology, Faculty of Veterinary Sciences, State University Utrecht, P.O. Box 80157, 3508 TD Utrecht, The Netherlands
P. C. Gogoi, Adviser Forests, (Wildlife and Environmental Planning), Meghalaya, P.O. Shillong 793 001, India
C. P. Groves, Department of Prehistory and Anthropology, The AustralianNationalUniversity, P.O. Box4, Canberra, ACT 2600, Australia
Alastair A. Macdonald, Department of Anatomy, Royal (Dick) School of Veterinary Studies, University of Edinburgh, Edinburgh EH9 lOH, Scotland, United Kingdom
J. MacKinnon, WWF/IUCN Representative, P.O. Box 133, Bogor, Indonesia
W. L. R. Oliver, Jersey Wildlife Preservation Trust, Les Augres Manor, Jersey, Channel Islands, United Kingdom
S. Deb Roy, Manas National Park, P.O. Barpeta Road, Kamrup 781 315, India
Zoos with Pigmy Hog
Gauhati, India (Assam State Zoo)
Zurich, Switzerland
Babirusa
A. A. Bosma, Department of Functional Morphology, Faculty of Veterinary Sciences, State University Utrecht, P.O. Box 80157, 3508 TD Utrecht, The Netherlands
C. P. Groves, Department of Prehistory and Anthropology, The Australian National University, P.O. Box 4, Canberra, ACT 2600, Australia
Alastair A. Macdonald, Department of Anatomy, Royal (Dick) School of Veterinary Studies, University of Edinburgh, Edinburgh EH9 lOH, Scotland, United Kingdom
J. MacKinnon, WWF/IUCN Representative, P.O. Box 133, Bogor, Indonesia
W. L. R. Oliver, Jersey Wildlife Preservation Trust, Les Augres Manor, Jersey, Channel Islands, United Kingdom
Victoria Selmier, 353 10th Street, San Francisco, California 94103, USA
Zoos with Babirusa
Antwerp, Belgium (Royal Zoological Society of Antwerp, W. de Meurichy)
Bandung, Indonesia
Berlin, Federal Republic of Germany, (Zoologischer Garten)
Copenhagen, Denmark, (Zoologisk Have)
Frankfurt, Federal Republic of Germany
Jakarta, Indonesia, (Kebun Binatang Ragunan)
Nuremberg, Federal Republic of Germany
Poznan, Poland
Rotterdam, The Netherlands (Stichting Koninklijke Rotterdamse Diergaarde)
Stuttgart, Federal Republic of Germany
Surabaya, Indonesia, (Kebun Binatang Surabaya)
HUGH POPENOE, Director, International Programs in Agriculture, University of Florida, Gainesville, Florida, Chairman
Members
WILLIAM BRADLEY, Consultant, New Hope, Pennsylvania
HAROLD DREGNE, Director, International Center for Arid and Semi-Arid Land Studies, Texas Tech University, Lubbock, Texas (member through 1981)
ELMER L. GADEN, JR., Department of Chemical Engineering, University of Virginia, Charlottesville, Virginia
CARL N. HODGES, Director, Environmental Research Laboratory, Tucson, Arizona
CYRUS MCKELL, Native Plants, Inc., Salt Lake City, Utah
FRANCOlS MERGEN, Pinchot Professor of Forestry, School of Forestry and Environmental Studies, Yale University, New Haven, Connecticut (member through 1982)
DONALD L. PLUCKNETT, Consultative Group on International Agricultural Research, Washington, D.C.
THEODORE SUDIA, Deputy Science Advisor to the Secretary of Interior, Department of Interior, Washington, D.C.
GEORGE BUGElARELLO, President, Polytechnic Institute of New York, Brooklyn, New York, Chairman
Members
SAMUEL P. ASPER, Educational Commission for Foreign Medical Graduates, Philadelphia, Pennsylvania
DAVID BELL, Department of Population Sciences, Harvard School of Public Health, Boston, Massachusetts
LEONARD BERRY, Professor, Graduate School of Geography, Clark University, Worcester, Massachusetts
ERNEST.J. BRISKEY, Dean, School of Agriculture, Oregon State University, Corvallis, Oregon
HARRISON S. BROWN, Director, Resources Systems Institute, East-West Center, Honolulu, Hawaii
ROBERT H. BURRIS, Department of Biochemistry, University of Wisconsin, Madison, Wisconsin
CLAUDIA JEAN CARR, Associate Professor, Conservation and Resource Studies, University of California at Berkeley, Berkeley, California
NATE FIELDS, Director, Developing Markets, Control Data Corporation, Minneapolis, Minnesota
ROLAND J. FUCHS, Chairman, Department of Geography, University of Hawaii at Manoa, Honolulu, Hawaii
ELMER L. GADEN, JR., Department of Chemical Engineering, University of Virginia, Charlottesville, Virginia
JOHN HOWARD GIBBONS, Director, U.S. Congress, Office of Technology Assessment, Washington, D.C.
N. BRUCE HANNAY, Foreign Secretary, National Academy of Engineering, Washington, D.C.
WILLIAM HUGHES, Director, Engineering Energy Laboratory, Oklahoma State University, Stillwater, Oklahoma
WILLIAM A. W. KREBS, Vice President, Arthur D. Little, Inc., Acorn Park, Cambridge, Massachusetts
GEORGE I. LYTHCOTT, University of Wisconsin, School of Medicine, Madison, Wisconsin
JANICE E. PERLMAN, Executive Director, Committee for a New New York, New York City Partnership, New York, New York
HUGH POPENOE, Director, International Programs in Agriculture, University of Florida, Gainesville, Florida
FREDERICK C. ROBBINS, President, Institute of Medicine, National Academy of Sciences, Washington, D.C.
WALTER A. ROSENBLITH, Foreign Secretary, National Academy of Sciences, Washington, D.C.
FREDERICK SEITZ, President Emeritus, The Rockefeller University, New York, New York
RALPH HERBERT SMUCKLER, Dean of International Studies and Programs, Michigan State University, East Lansing, Michigan
GILBERT F. WHITE, Institute of Behavioral Science, University of Colorado, Boulder, Colorado
BILL C. WRIGHT, Assistant Dean for International Programs, Oklahoma State University, Stillwater, Oklahoma
JOHN G. HURLEY, Director
MICHAEL G. C. McDONALD DOW, Associate Director/Studies
MICHAEL P. GREENE. Associate Director/Research Grants